Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.jprot.2020.103650 Ke-Ke Li 1 , De-Hui Qu 2 , Hai-Nan Zhang 3 , Fei-Yan Chen 1 , Lei Xu 1 , Meng-Yun Wang 1 , Hong-Yan Su 1 , Sheng-Ce Tao 3 , Fan-Lin Wu 1
|
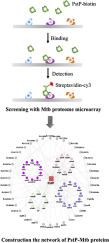
Mycobacterium tuberculosis (Mtb) serine/threonine protein phosphatase PstP plays an important role in regulating Mtb cell division and growth by reversible phosphorylation signaling. However, the substrates of Mtb with which the PstP interacts, and the underlying molecular mechanisms are still largely unknown. In this study, we performed an Mtb proteome microarray to globally identify the PstP bindings. In this way, we discovered 78 interactors between PstP and Mtb proteins, and found a novel connections with EthR. The interaction between PstP and EthR has been validated by Bio-Layer interferometry and Yeast-two-hybrid. And functional studies showed that PstP significantly enhances the binding between EthR and related DNA domain through its interaction with EthR. Phenotypically, overexpression of PstP promoted the resistance of Mycobacterium smegmatis with the antibiotic of ethionamide. Overall, we hopefully wish that the PstP interactors identified in this study will serve as a useful resource for further systematic studies of the roles that PstP plays in the regulation of Mtb dephosphorylation.
Significance
Mycobacterium tuberculosis (Mtb) is the causative agent of tuberculosis, which is responsible of ~1.5 million death per year. Understanding the knowledge about the basic biological regulation pathways in Mtb is an effective approach to discover the novel drug targets for cure TB. PstP is a serine/threonine protein phosphatase in Mtb, and plays important roles in regulating Mtb cell division and growth by reversible phosphorylation signaling. In this study, we identified 78 PstP interacting Mtb proteins using Mtb proteome microarray, which could preliminarily explain the roles of PstP played in Mtb. Moreover, functional analysis showed that a novel transcription factor EthR had been found regulated by PstP through binding, which could enhance the resistance to the antibiotic ETH. Overall, this network constructed with PstP-Mtb proteins could serve as a valuable resource for studying Mtb growth.
中文翻译:

全球使用Mtb蛋白质组微阵列发现PstP相互作用,并揭示了与EthR的新颖联系。
结核分枝杆菌(Mtb)丝氨酸/苏氨酸蛋白磷酸酶PstP通过可逆的磷酸化信号传导在调节Mtb细胞分裂和生长中起重要作用。然而,与PstP相互作用的Mtb的底物,以及潜在的分子机制仍是很大程度上未知。在这项研究中,我们进行了Mtb蛋白质组微阵列,以全面鉴定PstP结合。这样,我们发现了PstP和Mtb蛋白之间的78个相互作用因子,并发现了与EthR的新型连接。PstP和EthR之间的相互作用已通过Bio-Layer干涉法和酵母双杂交法得到验证。功能研究表明,PstP通过与EthR相互作用而显着增强EthR与相关DNA结构域之间的结合。从表型上看,PstP的过表达促进了PstP的抗性。耻垢分枝杆菌与乙硫酰胺的抗生素。总体而言,我们希望本研究中确定的PstP相互作用体将成为进一步系统研究PstP在Mtb脱磷酸调节中所起作用的有用资源。
意义
结核分枝杆菌(Mtb)是结核病的病原体,每年造成约150万人死亡。了解有关Mtb中基本生物学调控途径的知识是发现治愈TB的新型药物靶标的有效方法。PstP是Mtb中的丝氨酸/苏氨酸蛋白磷酸酶,并通过可逆的磷酸化信号在调节Mtb细胞分裂和生长中起重要作用。在这项研究中,我们使用Mtb蛋白质组微阵列鉴定了78种PstP相互作用的Mtb蛋白,这可以初步解释PstP在Mtb中发挥的作用。此外,功能分析表明,已发现一种新的转录因子EthR通过结合而受PstP调节,这可以增强对抗生素ETH的抗性。总体,










































 京公网安备 11010802027423号
京公网安备 11010802027423号