当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption system for Mg2+ removal from aqueous solutions using bentonite/γ-alumina nanocomposite.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.jcis.2020.01.036 Saeide Pourshadlou 1 , Iman Mobasherpour 1 , Houdsa Majidian 1 , Esmaeil Salahi 1 , Fatemeh Shirani Bidabadi 2 , Chang-Tong Mei 3 , Mohsen Ebrahimi 4
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.jcis.2020.01.036 Saeide Pourshadlou 1 , Iman Mobasherpour 1 , Houdsa Majidian 1 , Esmaeil Salahi 1 , Fatemeh Shirani Bidabadi 2 , Chang-Tong Mei 3 , Mohsen Ebrahimi 4
Affiliation
|
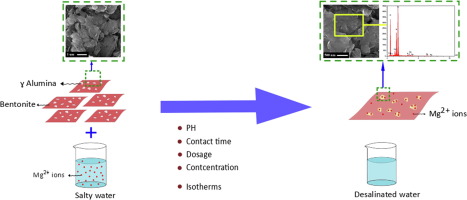
Calcium and magnesium are the most common sources of water hardness. These divalent ions can react with soap anions decreasing the cleaning efficiency and hence, high consumption of detergents occurred as a result. Development of novel low-cost adsorbents for metals removal has attracted a great attention. In this study, bentonite/γ-alumina nanocomposites were used to remove Mg2+ from water. Effects of process parameters including γ-alumina content, initial ion concentration, adsorbent dosage, contact time and pH on adsorption process were investigated. Increasing the amount of alumina in composite from 1 to 3 and 5 wt%, caused a negative effect on the amount of adsorbed magnesium ions per gram of adsorbent; while increasing the initial ion concentration from 60 ppm to 100 ppm resulted in higher uptake per unit mass of the adsorbent from 2.15 mg/g to 2.80 mg/g, respectively. Langmuir, Freundlich and D-K-R isotherm models were used for data analysis, among which the Langmuir model was found to be more successful (R2 = 0.9955), obtaining the maximum adsorption capacity (Qm) of 3.478 mg/g. Moreover, calculation of the adsorption energy (E) from DKR isotherm model depicted the physical nature of the adsorption of Mg2+ onto bentonite/γ-alumina nanocomposite powder.
中文翻译:

使用膨润土/γ-氧化铝纳米复合材料从水溶液中去除Mg2 +的吸附系统。
钙和镁是最常见的水硬度来源。这些二价离子可与肥皂阴离子反应,从而降低清洁效率,因此导致洗涤剂的大量消耗。用于去除金属的新型低成本吸附剂的开发引起了极大的关注。在这项研究中,膨润土/γ-氧化铝纳米复合材料用于去除水中的Mg2 +。研究了工艺参数,包括γ-氧化铝含量,初始离子浓度,吸附剂用量,接触时间和pH值对吸附过程的影响。复合材料中氧化铝的含量从1增加到3和5 wt%,会对每克吸附剂吸附的镁离子量产生负面影响;同时将初始离子浓度从60 ppm增加到100 ppm会使单位质量吸附剂的吸收量从2增加。15 mg / g至2.80 mg / g。使用Langmuir,Freundlich和DKR等温线模型进行数据分析,其中Langmuir模型更成功(R2 = 0.9955),最大吸附容量(Qm)为3.478 mg / g。此外,根据DKR等温模型计算的吸附能(E)描述了Mg2 +在膨润土/γ-氧化铝纳米复合粉体上的吸附物理性质。
更新日期:2020-01-17
中文翻译:

使用膨润土/γ-氧化铝纳米复合材料从水溶液中去除Mg2 +的吸附系统。
钙和镁是最常见的水硬度来源。这些二价离子可与肥皂阴离子反应,从而降低清洁效率,因此导致洗涤剂的大量消耗。用于去除金属的新型低成本吸附剂的开发引起了极大的关注。在这项研究中,膨润土/γ-氧化铝纳米复合材料用于去除水中的Mg2 +。研究了工艺参数,包括γ-氧化铝含量,初始离子浓度,吸附剂用量,接触时间和pH值对吸附过程的影响。复合材料中氧化铝的含量从1增加到3和5 wt%,会对每克吸附剂吸附的镁离子量产生负面影响;同时将初始离子浓度从60 ppm增加到100 ppm会使单位质量吸附剂的吸收量从2增加。15 mg / g至2.80 mg / g。使用Langmuir,Freundlich和DKR等温线模型进行数据分析,其中Langmuir模型更成功(R2 = 0.9955),最大吸附容量(Qm)为3.478 mg / g。此外,根据DKR等温模型计算的吸附能(E)描述了Mg2 +在膨润土/γ-氧化铝纳米复合粉体上的吸附物理性质。







































 京公网安备 11010802027423号
京公网安备 11010802027423号