当前位置:
X-MOL 学术
›
Colloids Surf. B Biointerfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH-responsive cationic liposome for endosomal escape mediated drug delivery.
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.colsurfb.2020.110804 Sagar Rayamajhi 1 , Jessica Marchitto 2 , Tuyen Duong Thanh Nguyen 1 , Ramesh Marasini 1 , Christian Celia 3 , Santosh Aryal 1
Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.colsurfb.2020.110804 Sagar Rayamajhi 1 , Jessica Marchitto 2 , Tuyen Duong Thanh Nguyen 1 , Ramesh Marasini 1 , Christian Celia 3 , Santosh Aryal 1
Affiliation
|
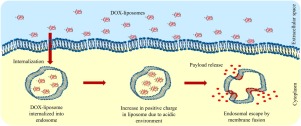
Endosomal degradation of the nanoparticle is one of the major biological barriers associated with the drug delivery system. Nanoparticles are internalized in the cell via different endocytosis pathways, where they are first delivered to early endosomes which mature to the late endosome and to the lysosome. During this journey, NP encounters a harsh chemical environment resulting in the degradation of NP and its content. This process is collectively called as intracellular defenses against foreign materials. Therefore, to avoid this degradative fate, the endosomal escape technique has been explored following membrane fusion or membrane destabilization mechanisms. However, these methods are limited to the application due to non-specific membrane fusion. To overcome this limitation, we have designed pH-responsive liposome made up of 3ß-[N-(N',N'-dimethylaminoethane)-carbamoyl]cholesterol hydrochloride (DC-liposome) in which the cationic nitrogen of the ammonium moiety occupies only ∼2.5 % of the molecule. Such a small percentage of the cationic moiety is sufficient enough to exhibit pH-responsive properties while maintaining the biocompatibility of the DC-liposome. DC-liposome showed pH-dependent cationic properties due to the protonation of DC-moiety at acidic pH. The fluorescence-based experiment confirmed pH-dependent fusogenic properties of DC-liposome. Furthermore, the endosomal colocalization study revealed higher localization of DC-liposome in the early endosome compared to that of the late endosome, suggesting possible endosomal escape. Elevated cationic and fusogenic properties of DC-liposome at acidic pH can mediate membrane fusion with anionic endosomal membrane via electrostatic interaction, thereby causing endosomal escape. Moreover, doxorubicin-loaded DC-liposome showed higher cytotoxicity than that of free doxorubicin further supporting our clam of endosomal escape. These findings suggest the potential of DC-liposome to break the endosomal barriers to enhance the therapeutic efficacy thereby guiding us in design consideration in the field of stimuli-responsive delivery agents.
中文翻译:

pH响应型阳离子脂质体用于内体逸出介导的药物递送。
纳米颗粒的内体降解是与药物递送系统相关的主要生物学障碍之一。纳米颗粒通过不同的内吞途径在细胞内被内化,在此它们首先被递送到早期的内体,后者逐渐成熟到晚期的内体和溶酶体。在此过程中,NP遇到恶劣的化学环境,导致NP及其含量下降。此过程统称为细胞内防御异物的防御措施。因此,为了避免这种降解的命运,人们在膜融合或膜不稳定机制之后探索了内体逃逸技术。但是,由于非特异性膜融合,这些方法仅限于应用。为克服这一限制,我们设计了由3ß-[N-(N',N'-二甲基氨基乙烷)-氨基甲酰基]胆固醇盐酸盐(DC-脂质体),其中铵部分的阳离子氮仅占分子的约2.5%。如此小的百分比的阳离子部分足以显示出pH响应特性,同时保持DC脂质体的生物相容性。DC脂质体由于在酸性pH下DC部分的质子化而显示出pH依赖性阳离子性质。基于荧光的实验证实了DC脂质体的pH依赖性融合特性。此外,内体共定位研究表明,与晚期内体相比,早期内体中DC-脂质体的定位更高,表明可能存在内体逃逸。DC脂质体在酸性pH下的高阳离子和融合特性可以通过静电相互作用介导与阴离子内体膜的膜融合,从而引起内体逸出。此外,负载阿霉素的DC脂质体比游离阿霉素具有更高的细胞毒性,进一步支持了我们的内体逃逸蛤。这些发现表明DC脂质体突破内体屏障以增强治疗功效的潜力,从而指导我们在刺激响应性递送剂领域的设计考虑。
更新日期:2020-01-17
中文翻译:

pH响应型阳离子脂质体用于内体逸出介导的药物递送。
纳米颗粒的内体降解是与药物递送系统相关的主要生物学障碍之一。纳米颗粒通过不同的内吞途径在细胞内被内化,在此它们首先被递送到早期的内体,后者逐渐成熟到晚期的内体和溶酶体。在此过程中,NP遇到恶劣的化学环境,导致NP及其含量下降。此过程统称为细胞内防御异物的防御措施。因此,为了避免这种降解的命运,人们在膜融合或膜不稳定机制之后探索了内体逃逸技术。但是,由于非特异性膜融合,这些方法仅限于应用。为克服这一限制,我们设计了由3ß-[N-(N',N'-二甲基氨基乙烷)-氨基甲酰基]胆固醇盐酸盐(DC-脂质体),其中铵部分的阳离子氮仅占分子的约2.5%。如此小的百分比的阳离子部分足以显示出pH响应特性,同时保持DC脂质体的生物相容性。DC脂质体由于在酸性pH下DC部分的质子化而显示出pH依赖性阳离子性质。基于荧光的实验证实了DC脂质体的pH依赖性融合特性。此外,内体共定位研究表明,与晚期内体相比,早期内体中DC-脂质体的定位更高,表明可能存在内体逃逸。DC脂质体在酸性pH下的高阳离子和融合特性可以通过静电相互作用介导与阴离子内体膜的膜融合,从而引起内体逸出。此外,负载阿霉素的DC脂质体比游离阿霉素具有更高的细胞毒性,进一步支持了我们的内体逃逸蛤。这些发现表明DC脂质体突破内体屏障以增强治疗功效的潜力,从而指导我们在刺激响应性递送剂领域的设计考虑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号