当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Regulation of Circadian Chromatin.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.jmb.2020.01.009 Qiaoqiao Zhu 1 , William J Belden 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.jmb.2020.01.009 Qiaoqiao Zhu 1 , William J Belden 1
Affiliation
|
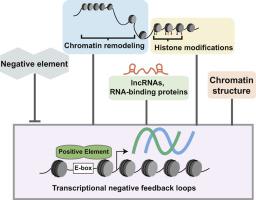
Circadian rhythms are generated by transcriptional negative feedback loops and require histone modifications and chromatin remodeling to ensure appropriate timing and amplitude of clock gene expression. Circadian modifications to histones are important for transcriptional initiation and feedback inhibition serving as signaling platform for chromatin-remodeling enzymes. Current models indicate circadian-regulated facultative heterochromatin (CRFH) is a conserved mechanism at clock genes in Neurospora, Drosophila, and mice. CRFH consists of antiphasic rhythms in activating and repressive modifications generating chromatin states that cycle between transcriptionally permissive and nonpermissive. There are rhythms in histone H3 lysine 9 and 27 acetylation (H3K9ac and H3K27ac) and histone H3 lysine 4 methylation (H3K4me) during activation; while deacetylation, histone H3 lysine 9 methylation (H3K9me) and heterochromatin protein 1 (HP1) are hallmarks of repression. ATP-dependent chromatin-remodeling enzymes control accessibility, nucleosome positioning/occupancy, and nuclear organization. In Neurospora, the rhythm in facultative heterochromatin is mediated by the frequency (frq) natural antisense transcript (NAT) qrf. While in mammals, histone deacetylases (HDACs), histone H3 lysine 9 methyltransferase (KMT1/SUV39), and components of nucleosome remodeling and deacetylase (NuRD) are part of the nuclear PERIOD complex (PER complex). Genomics efforts have found relationships among rhythmic chromatin modifications at clock-controlled genes (ccg) revealing circadian control of genome-wide chromatin states. There are also circadian clock-regulated lncRNAs with an emerging function that includes assisting in chromatin dynamics. In this review, we explore the connections between circadian clock, chromatin remodeling, lncRNAs, and CRFH and how these impact rhythmicity, amplitude, period, and phase of circadian clock genes.
中文翻译:

昼夜节律染色质的分子调控。
昼夜节律是由转录负反馈环产生的,需要进行组蛋白修饰和染色质重塑,以确保时钟基因表达的适当时机和幅度。对组蛋白的昼夜节律修饰对于转录起始和反馈抑制至关重要,可作为染色质重塑酶的信号平台。当前模型表明,昼夜节律调节的兼性异染色质(CRFH)是Neurospora,果蝇和小鼠中时钟基因的保守机制。CRFH由激活和抑制修饰中的反相位节律组成,产生染色质状态,这些状态在转录允许和非允许之间循环。在激活过程中,组蛋白H3赖氨酸9和27的乙酰化(H3K9ac和H3K27ac)和组蛋白H3赖氨酸4甲基化(H3K4me)有节律。而去乙酰化时,组蛋白H3赖氨酸9甲基化(H3K9me)和异染色质蛋白1(HP1)是抑制的标志。ATP依赖的染色质重塑酶控制可及性,核小体定位/占有率和核组织。在Neurospora中,兼性异染色质的节律由频率(frq)自然反义转录本(NAT)qrf介导。在哺乳动物中,组蛋白脱乙酰基酶(HDAC),组蛋白H3赖氨酸9甲基转移酶(KMT1 / SUV39)以及核小体重塑和脱乙酰基酶(NuRD)的成分是核PERIOD复合物(PER复合物)的一部分。基因组学方面的努力已经发现时钟控制基因(ccg)的有节奏染色质修饰之间的关系,揭示了对全基因组染色质状态的昼夜控制。也有昼夜节律调节的lncRNA,具有新兴功能,包括协助染色质动力学。在这篇综述中,我们探讨了昼夜节律时钟,染色质重塑,lncRNA和CRFH之间的联系,以及这些因素如何影响昼夜节律时钟基因的节律,振幅,周期和相位。
更新日期:2020-01-16
中文翻译:

昼夜节律染色质的分子调控。
昼夜节律是由转录负反馈环产生的,需要进行组蛋白修饰和染色质重塑,以确保时钟基因表达的适当时机和幅度。对组蛋白的昼夜节律修饰对于转录起始和反馈抑制至关重要,可作为染色质重塑酶的信号平台。当前模型表明,昼夜节律调节的兼性异染色质(CRFH)是Neurospora,果蝇和小鼠中时钟基因的保守机制。CRFH由激活和抑制修饰中的反相位节律组成,产生染色质状态,这些状态在转录允许和非允许之间循环。在激活过程中,组蛋白H3赖氨酸9和27的乙酰化(H3K9ac和H3K27ac)和组蛋白H3赖氨酸4甲基化(H3K4me)有节律。而去乙酰化时,组蛋白H3赖氨酸9甲基化(H3K9me)和异染色质蛋白1(HP1)是抑制的标志。ATP依赖的染色质重塑酶控制可及性,核小体定位/占有率和核组织。在Neurospora中,兼性异染色质的节律由频率(frq)自然反义转录本(NAT)qrf介导。在哺乳动物中,组蛋白脱乙酰基酶(HDAC),组蛋白H3赖氨酸9甲基转移酶(KMT1 / SUV39)以及核小体重塑和脱乙酰基酶(NuRD)的成分是核PERIOD复合物(PER复合物)的一部分。基因组学方面的努力已经发现时钟控制基因(ccg)的有节奏染色质修饰之间的关系,揭示了对全基因组染色质状态的昼夜控制。也有昼夜节律调节的lncRNA,具有新兴功能,包括协助染色质动力学。在这篇综述中,我们探讨了昼夜节律时钟,染色质重塑,lncRNA和CRFH之间的联系,以及这些因素如何影响昼夜节律时钟基因的节律,振幅,周期和相位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号