当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Co-delivery of 2-Deoxyglucose and a glutamine metabolism inhibitor V9302 via a prodrug micellar formulation for synergistic targeting of metabolism in cancer.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.actbio.2020.01.019 Zhangyi Luo 1 , Jieni Xu 1 , Jingjing Sun 1 , Haozhe Huang 1 , Ziqian Zhang 1 , Weina Ma 1 , Zhuoya Wan 1 , Yangwuyue Liu 1 , Apurva Pardeshi 1 , Song Li 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.actbio.2020.01.019 Zhangyi Luo 1 , Jieni Xu 1 , Jingjing Sun 1 , Haozhe Huang 1 , Ziqian Zhang 1 , Weina Ma 1 , Zhuoya Wan 1 , Yangwuyue Liu 1 , Apurva Pardeshi 1 , Song Li 1
Affiliation
|
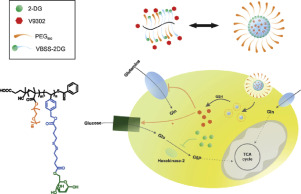
The unique metabolic demand of cancer cells suggests a new therapeutic strategy targeting the metabolism in cancers. V9302 is a recently reported inhibitor of ASCT2 amino acid transporter which shows promising antitumor activity by blocking glutamine uptake. However, its poor solubility in aqueous solutions and tumor cells' compensatory metabolic shift to glucose metabolism may limit the antitumor efficacy of V9302. 2-Deoxyglucose (2-DG), a derivative of glucose, has been developed as a potential antitumor agent through inhibiting glycolysis in tumor cells. In order to achieve enhanced antitumor effect by inhibiting both metabolic pathways, a 2-DG prodrug-based micellar carrier poly-(oligo ethylene glycol)-co-poly(4-((4-oxo-4-((4-vinylbenzyl)oxy)butyl)disulfaneyl)butanoic acid)-(2-deoxyglucose) (POEG-p-2DG) was developed. POEG-p-2DG well retained the pharmacological activity of 2-DG in vitro and in vivo, More importantly, POEG-p-2DG could self-assemble to form micelles that were capable of loading V9302 to achieve co-delivery of 2-DG and V9302. V9302-loaded POEG-p2DG micelles were small in sizes (~10 nm), showed a slow kinetics of drug release and demonstrated targeted delivery to tumor. In addition, V9302 loaded POEG-p-2DG micelles exhibited improved anti-tumor efficacy both in vitro and in vivo. Interestingly, 2-DG treatment further decreased the glutamine uptake when combined with V9302, likely due to inhibition of ASCT2 glycosylation. These results suggest that POEG-p2DG prodrug micelles may serve as a dual functional carrier for V9302 to achieve synergistic targeting of metabolism in cancers. STATEMENT OF SIGNIFICANCE: Unique cancer cell's metabolism profile denotes a new therapeutic strategy. V9302 is a recently reported glutamine metabolism inhibitor that shows promising antitumor activity. However, its poor waster solubility and tumor cell's compensatory metabolic network may limit its potential clinical application. 2-Deoxyglucose(2-DG) is a widely used glycolysis inhibitor. However, its clinical application is hindered by low efficacy as monotherapy. Thus, in this study, we developed a redox-sensitive, 2-DG-based prodrug polymer, as a dual-functional carrier for co-delivery of V9302 and 2-DG as a combination strategy. V9302 loaded POEG-p-2DG micelle showed significantly improved antitumor activity through synergistic targeting of both glutamine and glycolysis metabolism pathway. More interestingly, POEG-p-2DG itself further facilitates inhibition of glutamine metabolism, likely through inhibition of ASCT2 glycosylation.
中文翻译:

通过前药胶束制剂共同递送 2-脱氧葡萄糖和谷氨酰胺代谢抑制剂 V9302,以协同靶向癌症代谢。
癌细胞独特的代谢需求提出了一种针对癌症代谢的新治疗策略。V9302 是最近报道的 ASCT2 氨基酸转运蛋白抑制剂,通过阻断谷氨酰胺的摄取而显示出有前景的抗肿瘤活性。然而,其在水溶液中的溶解度差以及肿瘤细胞代偿性代谢转变为葡萄糖代谢可能会限制V9302的抗肿瘤功效。2-脱氧葡萄糖 (2-DG) 是葡萄糖的衍生物,通过抑制肿瘤细胞的糖酵解而被开发为潜在的抗肿瘤剂。为了通过抑制两种代谢途径来增强抗肿瘤效果,基于2-DG前药的胶束载体聚-(低聚乙二醇)-共-聚(4-((4-oxo-4-((4-vinylbenzyl))开发了氧)丁基)二硫基)丁酸)-(2-脱氧葡萄糖)(POEG-p-2DG)。POEG-p-2DG在体外和体内都很好地保留了2-DG的药理活性,更重要的是,POEG-p-2DG可以自组装形成能够负载V9302的胶束,从而实现2-DG的共递送和V9302。负载 V9302 的 POEG-p2DG 胶束尺寸较小(~10 nm),显示出缓慢的药物释放动力学,并被证明可以靶向递送至肿瘤。此外,负载V9302的POEG-p-2DG胶束在体外和体内均表现出改善的抗肿瘤功效。有趣的是,当与 V9302 联合使用时,2-DG 治疗进一步降低了谷氨酰胺的摄取,这可能是由于 ASCT2 糖基化的抑制。这些结果表明 POEG-p2DG 前药胶束可以作为 V9302 的双重功能载体,以实现癌症代谢的协同靶向。意义声明:独特的癌细胞代谢特征代表了一种新的治疗策略。V9302 是最近报道的一种谷氨酰胺代谢抑制剂,显示出有前景的抗肿瘤活性。然而,其较差的废物溶解度和肿瘤细胞的代偿代谢网络可能限制其潜在的临床应用。2-脱氧葡萄糖(2-DG)是一种广泛使用的糖酵解抑制剂。然而,其单一疗法的疗效较低,阻碍了其临床应用。因此,在本研究中,我们开发了一种氧化还原敏感的基于 2-DG 的前药聚合物,作为双功能载体,用于共同递送 V9302 和 2-DG 作为组合策略。负载 V9302 的 POEG-p-2DG 胶束通过协同靶向谷氨酰胺和糖酵解代谢途径,显示出显着提高的抗肿瘤活性。更有趣的是,POEG-p-2DG 本身可能通过抑制 ASCT2 糖基化进一步促进谷氨酰胺代谢的抑制。
更新日期:2020-01-17
中文翻译:

通过前药胶束制剂共同递送 2-脱氧葡萄糖和谷氨酰胺代谢抑制剂 V9302,以协同靶向癌症代谢。
癌细胞独特的代谢需求提出了一种针对癌症代谢的新治疗策略。V9302 是最近报道的 ASCT2 氨基酸转运蛋白抑制剂,通过阻断谷氨酰胺的摄取而显示出有前景的抗肿瘤活性。然而,其在水溶液中的溶解度差以及肿瘤细胞代偿性代谢转变为葡萄糖代谢可能会限制V9302的抗肿瘤功效。2-脱氧葡萄糖 (2-DG) 是葡萄糖的衍生物,通过抑制肿瘤细胞的糖酵解而被开发为潜在的抗肿瘤剂。为了通过抑制两种代谢途径来增强抗肿瘤效果,基于2-DG前药的胶束载体聚-(低聚乙二醇)-共-聚(4-((4-oxo-4-((4-vinylbenzyl))开发了氧)丁基)二硫基)丁酸)-(2-脱氧葡萄糖)(POEG-p-2DG)。POEG-p-2DG在体外和体内都很好地保留了2-DG的药理活性,更重要的是,POEG-p-2DG可以自组装形成能够负载V9302的胶束,从而实现2-DG的共递送和V9302。负载 V9302 的 POEG-p2DG 胶束尺寸较小(~10 nm),显示出缓慢的药物释放动力学,并被证明可以靶向递送至肿瘤。此外,负载V9302的POEG-p-2DG胶束在体外和体内均表现出改善的抗肿瘤功效。有趣的是,当与 V9302 联合使用时,2-DG 治疗进一步降低了谷氨酰胺的摄取,这可能是由于 ASCT2 糖基化的抑制。这些结果表明 POEG-p2DG 前药胶束可以作为 V9302 的双重功能载体,以实现癌症代谢的协同靶向。意义声明:独特的癌细胞代谢特征代表了一种新的治疗策略。V9302 是最近报道的一种谷氨酰胺代谢抑制剂,显示出有前景的抗肿瘤活性。然而,其较差的废物溶解度和肿瘤细胞的代偿代谢网络可能限制其潜在的临床应用。2-脱氧葡萄糖(2-DG)是一种广泛使用的糖酵解抑制剂。然而,其单一疗法的疗效较低,阻碍了其临床应用。因此,在本研究中,我们开发了一种氧化还原敏感的基于 2-DG 的前药聚合物,作为双功能载体,用于共同递送 V9302 和 2-DG 作为组合策略。负载 V9302 的 POEG-p-2DG 胶束通过协同靶向谷氨酰胺和糖酵解代谢途径,显示出显着提高的抗肿瘤活性。更有趣的是,POEG-p-2DG 本身可能通过抑制 ASCT2 糖基化进一步促进谷氨酰胺代谢的抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号