当前位置:
X-MOL 学术
›
Kidney Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport's syndrome.
Kidney International ( IF 14.8 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.kint.2019.12.015 Oliver Gross 1 , Burkhard Tönshoff 2 , Lutz T Weber 3 , Lars Pape 4 , Kay Latta 5 , Henry Fehrenbach 6 , Baerbel Lange-Sperandio 7 , Hildegard Zappel 8 , Peter Hoyer 9 , Hagen Staude 10 , Sabine König 11 , Ulrike John 12 , Jutta Gellermann 13 , Bernd Hoppe 14 , Matthias Galiano 15 , Britta Hoecker 2 , Rasmus Ehren 3 , Christian Lerch 4 , Clifford E Kashtan 16 , Markus Harden 17 , Jan Boeckhaus 1 , Tim Friede 17 ,
Kidney International ( IF 14.8 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.kint.2019.12.015 Oliver Gross 1 , Burkhard Tönshoff 2 , Lutz T Weber 3 , Lars Pape 4 , Kay Latta 5 , Henry Fehrenbach 6 , Baerbel Lange-Sperandio 7 , Hildegard Zappel 8 , Peter Hoyer 9 , Hagen Staude 10 , Sabine König 11 , Ulrike John 12 , Jutta Gellermann 13 , Bernd Hoppe 14 , Matthias Galiano 15 , Britta Hoecker 2 , Rasmus Ehren 3 , Christian Lerch 4 , Clifford E Kashtan 16 , Markus Harden 17 , Jan Boeckhaus 1 , Tim Friede 17 ,
Affiliation
|
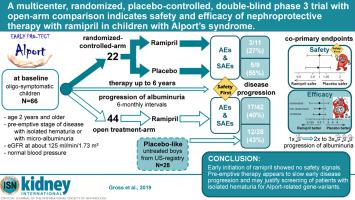
Children with Alport syndrome develop renal failure early in life. Since the safety and efficacy of preemptive nephroprotective therapy are uncertain we conducted a randomized, placebo-controlled, double-blind trial in 14 German sites of pediatric patients with ramipril for three to six years plus six months follow-up to determine these parameters. Pretreated children and those whose parents refused randomization became an open-arm control, which were compared to prospective real-world data from untreated children. The co-primary endpoints were safety (adverse drug reactions) and efficacy (time to progression). Out of 66 oligosymptomatic children, 22 were randomized and 44 joined the open-arm comparison. Ramipril therapy showed no safety issues (total of 216.4 patient-years on ramipril; adverse event rate-ratio 1.00; 95% confidence interval 0.66-1.53). Although not significant, our results cautiously showed that ramipril therapy was effective: in the randomized arm, Ramipril decreased the risk of disease progression by almost half (hazard ratio 0.51 (0.12-2.20)), diminished the slope of albuminuria progression and the decline in glomerular filtration. In adjusted analysis, indications of efficacy were supported by prospective data from participants treated open label compared with untreated children, in whom ramipril again seemed to reduce progression by almost half (0.53 (0.22-1.29)). Incorporating these results into the randomized data by Bayesian evidence synthesis resulted in a more precise estimate of the hazard-ratio of 0.52 (0.19-1.39). Thus, our study shows the safety of early initiation of therapy and supports the hope to slow renal failure by many years, emphasizing the value of preemptive therapy. Hence, screening programs for glomerular hematuria in children and young adults could benefit from inclusion of genetic testing for Alport-related gene-variants.
中文翻译:

一项多臂,随机,安慰剂对照,双盲的3期开放试验比较试验表明,雷米普利对阿尔波特综合症儿童进行肾保护疗法的安全性和有效性。
患有Alport综合征的儿童在生命早期发展为肾衰竭。由于尚不确定先发性肾脏保护疗法的安全性和有效性,因此我们在德国的雷米普利儿科患者的14个地点进行了一项随机,安慰剂对照,双盲试验,进行了三到六年以及六个月的随访以确定这些参数。将经过预处理的孩子和父母拒绝随机分配的孩子变成了开放式对照组,将其与未经治疗的孩子的前瞻性现实世界数据进行了比较。共同的主要终点是安全性(药物不良反应)和疗效(进展时间)。在66名有症状的少儿中,有22名被随机分组,有44名参加了开放式比较。雷米普利治疗未显示安全性问题(雷米普利总计216.4患者年;不良事件发生率-1.00; 95%置信区间0.66-1。53)。尽管不显着,但我们的结果谨慎地显示了雷米普利治疗有效:在随机分组中,雷米普利将疾病进展的风险降低了近一半(危险比0.51(0.12-2.20)),减少了蛋白尿进展的斜率并降低了肾小球滤过。在调整后的分析中,与未治疗的儿童相比,接受开放标签治疗的参与者的前瞻性数据支持了疗效指标,雷米普利似乎再次降低了近一半的进展(0.53(0.22-1.29))。通过贝叶斯证据合成将这些结果纳入随机数据中,可以得出0.52(0.19-1.39)的危险比的更精确估计。因此,我们的研究表明了及早开始治疗的安全性,并支持了将肾衰减缓多年的希望,强调抢先治疗的价值。因此,儿童和年轻人肾小球性血尿的筛查程序可能会受益于Alport相关基因变体的基因检测。
更新日期:2020-01-17
中文翻译:

一项多臂,随机,安慰剂对照,双盲的3期开放试验比较试验表明,雷米普利对阿尔波特综合症儿童进行肾保护疗法的安全性和有效性。
患有Alport综合征的儿童在生命早期发展为肾衰竭。由于尚不确定先发性肾脏保护疗法的安全性和有效性,因此我们在德国的雷米普利儿科患者的14个地点进行了一项随机,安慰剂对照,双盲试验,进行了三到六年以及六个月的随访以确定这些参数。将经过预处理的孩子和父母拒绝随机分配的孩子变成了开放式对照组,将其与未经治疗的孩子的前瞻性现实世界数据进行了比较。共同的主要终点是安全性(药物不良反应)和疗效(进展时间)。在66名有症状的少儿中,有22名被随机分组,有44名参加了开放式比较。雷米普利治疗未显示安全性问题(雷米普利总计216.4患者年;不良事件发生率-1.00; 95%置信区间0.66-1。53)。尽管不显着,但我们的结果谨慎地显示了雷米普利治疗有效:在随机分组中,雷米普利将疾病进展的风险降低了近一半(危险比0.51(0.12-2.20)),减少了蛋白尿进展的斜率并降低了肾小球滤过。在调整后的分析中,与未治疗的儿童相比,接受开放标签治疗的参与者的前瞻性数据支持了疗效指标,雷米普利似乎再次降低了近一半的进展(0.53(0.22-1.29))。通过贝叶斯证据合成将这些结果纳入随机数据中,可以得出0.52(0.19-1.39)的危险比的更精确估计。因此,我们的研究表明了及早开始治疗的安全性,并支持了将肾衰减缓多年的希望,强调抢先治疗的价值。因此,儿童和年轻人肾小球性血尿的筛查程序可能会受益于Alport相关基因变体的基因检测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号