Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Treatment patterns among patients with moderate-to-severe ulcerative colitis in the United States and Europe.
PLOS ONE ( IF 2.9 ) Pub Date : 2020-01-16 , DOI: 10.1371/journal.pone.0227914 Alessandro Armuzzi 1 , Marco daCosta DiBonaventura 2 , Miriam Tarallo 3 , James Lucas 4 , Daniel Bluff 4 , Benjamin Hoskin 4 , Danielle Bargo 2 , Joseph C Cappelleri 5 , Daniel Quirk 6 , Leonardo Salese 6
PLOS ONE ( IF 2.9 ) Pub Date : 2020-01-16 , DOI: 10.1371/journal.pone.0227914 Alessandro Armuzzi 1 , Marco daCosta DiBonaventura 2 , Miriam Tarallo 3 , James Lucas 4 , Daniel Bluff 4 , Benjamin Hoskin 4 , Danielle Bargo 2 , Joseph C Cappelleri 5 , Daniel Quirk 6 , Leonardo Salese 6
Affiliation
|
OBJECTIVE
The aim of the present study is to examine how moderate-to-severe ulcerative colitis (UC) is currently managed in real-world clinical practice across the United States (US) and European Union Five (EU5; France, Germany, Italy, Spain, and the United Kingdom).
METHODS
Data from the 2017 Adelphi Inflammatory Bowel-Disease Specific Programme (IBD-DSP) were used. The IBD-DSP is a database of patient chart information abstracted by selected gastroenterologists across the US and EU5. Eligible gastroenterologists who agreed to participate were asked to complete patient record forms for the next seven consecutive eligible adult patients with UC. Only charts from patients with moderate-to-severe UC were included in the analysis (defined as those with documented administration of either an immunosuppressant [IM] or a biologic). Treatment patterns were reported descriptively.
RESULTS
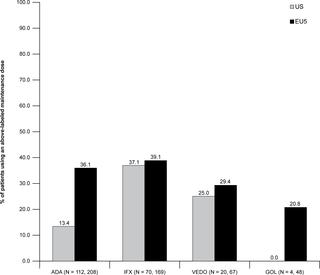
411 and 1191 patient charts were included in the US and EU5 (mean ages 44.2 and 39.6 years; 53.0% and 43.5% female), respectively. For those with complete treatment history, 40.7% and 52.9% used either an IM or biologic as their first treatment (with or without steroids). Usage of these therapies increased in subsequent lines. The percentage of patients treated with combination therapy (i.e., biologic therapy with a concomitant IM) in first line generally varied between 10-20% (e.g., US: adalimumab (ADA), 10.8%; infliximab (IFX), 18.2%; EU5: ADA, 12.5%; IFX, 19.9%), though increased in later lines in the EU5. Among patients currently using a biologic therapy, between 10-40% of patients used a higher than indicated dose or greater than indicated dosing frequency during maintenance (e.g., US: IFX, 37.1%; ADA, 13.4%; EU5: IFX, 39.1%; ADA, 36.1%). In both the US and EU5, the primary reason for switching therapy was efficacy-related.
CONCLUSIONS
In this analysis, many patients with moderate-to-severe UC use an IM or biologic as their first therapy after diagnosis. Combination therapy and dose escalation are also common, and underscore the challenges with managing this patient population.
中文翻译:

在美国和欧洲,中至重度溃疡性结肠炎患者的治疗方式。
目的本研究的目的是研究目前在美国(US)和欧盟五国(EU5),法国,德国,意大利,欧洲五国(EU5)的实际临床实践中,如何管理中度至重度溃疡性结肠炎(UC)西班牙和英国)。方法使用2017年阿德菲炎症性肠病特异性计划(IBD-DSP)的数据。IBD-DSP是由美国和EU5的部分胃肠病学家提取的患者病历信息数据库。同意参加的合格肠胃病医师要为接下来的连续七名合格的UC成人患者填写患者记录表。分析中仅包括来自中度至重度UC患者的图表(定义为已记录免疫抑制剂[IM]或生物制剂给药的图表)。描述性报道了治疗方式。结果411和1191个患者图表分别纳入美国和EU5(平均年龄44.2和39.6岁;女性分别为53.0%和43.5%)。对于具有完整治疗史的患者,有40.7%和52.9%的患者使用IM或生物制剂作为首次治疗(有或没有类固醇)。这些疗法的使用在随后的系中有所增加。一线接受联合疗法(即伴随IM的生物疗法)治疗的患者百分比通常在10%至20%之间变化(例如,美国:阿达木单抗(ADA),为10.8%;英夫利昔单抗(IFX),为18.2%; EU5 :ADA,12.5%; IFX,19.9%),但在EU5的更高版本中有所增加。在目前使用生物疗法的患者中,有10%至40%的患者在维持期间使用高于指示的剂量或高于指示的给药频率(例如,US:IFX,37.1%;ADA,13.4%;欧盟5:IFX,39.1%;ADA,36.1%)。在美国和欧盟5,转换治疗的主要原因是与疗效相关的。结论在此分析中,许多中至重度UC患者在诊断后将IM或生物制剂作为他们的第一疗法。组合疗法和剂量递增疗法也很常见,这凸显了管理该患者人群的挑战。
更新日期:2020-01-17
中文翻译:

在美国和欧洲,中至重度溃疡性结肠炎患者的治疗方式。
目的本研究的目的是研究目前在美国(US)和欧盟五国(EU5),法国,德国,意大利,欧洲五国(EU5)的实际临床实践中,如何管理中度至重度溃疡性结肠炎(UC)西班牙和英国)。方法使用2017年阿德菲炎症性肠病特异性计划(IBD-DSP)的数据。IBD-DSP是由美国和EU5的部分胃肠病学家提取的患者病历信息数据库。同意参加的合格肠胃病医师要为接下来的连续七名合格的UC成人患者填写患者记录表。分析中仅包括来自中度至重度UC患者的图表(定义为已记录免疫抑制剂[IM]或生物制剂给药的图表)。描述性报道了治疗方式。结果411和1191个患者图表分别纳入美国和EU5(平均年龄44.2和39.6岁;女性分别为53.0%和43.5%)。对于具有完整治疗史的患者,有40.7%和52.9%的患者使用IM或生物制剂作为首次治疗(有或没有类固醇)。这些疗法的使用在随后的系中有所增加。一线接受联合疗法(即伴随IM的生物疗法)治疗的患者百分比通常在10%至20%之间变化(例如,美国:阿达木单抗(ADA),为10.8%;英夫利昔单抗(IFX),为18.2%; EU5 :ADA,12.5%; IFX,19.9%),但在EU5的更高版本中有所增加。在目前使用生物疗法的患者中,有10%至40%的患者在维持期间使用高于指示的剂量或高于指示的给药频率(例如,US:IFX,37.1%;ADA,13.4%;欧盟5:IFX,39.1%;ADA,36.1%)。在美国和欧盟5,转换治疗的主要原因是与疗效相关的。结论在此分析中,许多中至重度UC患者在诊断后将IM或生物制剂作为他们的第一疗法。组合疗法和剂量递增疗法也很常见,这凸显了管理该患者人群的挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号