Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aggregation of CAT tails blocks their degradation and causes proteotoxicity in S. cerevisiae.
PLOS ONE ( IF 2.9 ) Pub Date : 2020-01-16 , DOI: 10.1371/journal.pone.0227841 Cole S Sitron 1 , Joseph H Park 1, 2 , Jenna M Giafaglione 1 , Onn Brandman 1
PLOS ONE ( IF 2.9 ) Pub Date : 2020-01-16 , DOI: 10.1371/journal.pone.0227841 Cole S Sitron 1 , Joseph H Park 1, 2 , Jenna M Giafaglione 1 , Onn Brandman 1
Affiliation
|
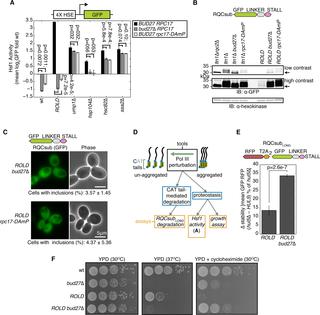
The Ribosome-associated Quality Control (RQC) pathway co-translationally marks incomplete polypeptides from stalled translation with two signals that trigger their proteasome-mediated degradation. The E3 ligase Ltn1 adds ubiquitin and Rqc2 directs the large ribosomal subunit to append carboxy-terminal alanine and threonine residues (CAT tails). When excessive amounts of incomplete polypeptides evade Ltn1, CAT-tailed proteins accumulate and can self-associate into aggregates. CAT tail aggregation has been hypothesized to either protect cells by sequestering potentially toxic incomplete polypeptides or harm cells by disrupting protein homeostasis. To distinguish between these possibilities, we modulated CAT tail aggregation in Saccharomyces cerevisiae with genetic and chemical tools to analyze CAT tails in aggregated and un-aggregated states. We found that enhancing CAT tail aggregation induces proteotoxic stress and antagonizes degradation of CAT-tailed proteins, while inhibiting aggregation reverses these effects. Our findings suggest that CAT tail aggregation harms RQC-compromised cells and that preventing aggregation can mitigate this toxicity.
中文翻译:

CAT尾巴的聚集会阻止其降解,并在酿酒酵母中引起蛋白毒性。
核糖体相关的质量控制(RQC)途径与两个触发其蛋白酶体介导的降解的信号共翻译,标记了来自停止翻译的不完整多肽。E3连接酶Ltn1添加泛素,Rqc2指导较大的核糖体亚基附加羧基末端的丙氨酸和苏氨酸残基(CAT尾巴)。当大量的不完全多肽逃逸到Ltn1时,CAT尾蛋白会积累并可以自缔合成聚集体。CAT尾巴聚集被认为可以通过隔离潜在的有毒不完整多肽来保护细胞,或者通过破坏蛋白质稳态来伤害细胞。为了区分这些可能性,我们使用遗传和化学工具调节了酿酒酵母中的CAT尾部聚集,以分析处于聚集和未聚集状态的CAT尾部。我们发现增强CAT尾部聚集可诱导蛋白毒性胁迫并拮抗CAT尾蛋白的降解,而抑制聚集可逆转这些作用。我们的发现表明,CAT尾部聚集会损害RQC受损的细胞,防止聚集可以减轻这种毒性。
更新日期:2020-01-17
中文翻译:

CAT尾巴的聚集会阻止其降解,并在酿酒酵母中引起蛋白毒性。
核糖体相关的质量控制(RQC)途径与两个触发其蛋白酶体介导的降解的信号共翻译,标记了来自停止翻译的不完整多肽。E3连接酶Ltn1添加泛素,Rqc2指导较大的核糖体亚基附加羧基末端的丙氨酸和苏氨酸残基(CAT尾巴)。当大量的不完全多肽逃逸到Ltn1时,CAT尾蛋白会积累并可以自缔合成聚集体。CAT尾巴聚集被认为可以通过隔离潜在的有毒不完整多肽来保护细胞,或者通过破坏蛋白质稳态来伤害细胞。为了区分这些可能性,我们使用遗传和化学工具调节了酿酒酵母中的CAT尾部聚集,以分析处于聚集和未聚集状态的CAT尾部。我们发现增强CAT尾部聚集可诱导蛋白毒性胁迫并拮抗CAT尾蛋白的降解,而抑制聚集可逆转这些作用。我们的发现表明,CAT尾部聚集会损害RQC受损的细胞,防止聚集可以减轻这种毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号