当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding Li‐Ion Dynamics in Lithium Hydroxychloride (Li2OHCl) Solid State Electrolyte via Addressing the Role of Protons
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-01-17 , DOI: 10.1002/aenm.201903480 Ah‐Young Song 1 , Kostiantyn Turcheniuk 1 , Johannes Leisen 2 , Yiran Xiao 1 , Lamartine Meda 3 , Oleg Borodin 4 , Gleb Yushin 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-01-17 , DOI: 10.1002/aenm.201903480 Ah‐Young Song 1 , Kostiantyn Turcheniuk 1 , Johannes Leisen 2 , Yiran Xiao 1 , Lamartine Meda 3 , Oleg Borodin 4 , Gleb Yushin 1
Affiliation
|
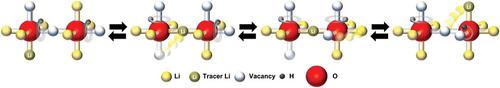
Low‐melting‐point solid‐state electrolytes (SSE) are critically important for low‐cost manufacturing of all‐solid‐state batteries. Lithium hydroxychloride (Li2OHCl) is a promising material within the SSE domain due to its low melting point (mp < 300 °C), cheap ingredients (Li, H, O, and Cl), and rapid synthesis. Another unique feature of this compound is the presence of Li vacancies and rotating hydroxyl groups which promote Li‐ion diffusion, yet the role of the protons in the ion transport remains poorly understood. To examine lithium and proton dynamics, a set of solid‐state NMR experiments are conducted, such as magic‐angle spinning 7Li NMR, static 7Li and 1H NMR, and spin‐lattice T1(7Li)/T1(1H) relaxation experiments. It is determined that only Li+ contributes to long‐range ion transport, while H+ dynamics is constrained to an incomplete isotropic rotation of the OH group. The results uncover detailed mechanistic understanding of the ion transport in Li2OHCl. It is shown that two distinct phases of ionic motions appear at low and elevated temperatures, and that the rotation of the OH group controls Li+ and H+ dynamics in both phases. The model based on the NMR experiments is fully consistent with crystallographic information, ionic conductivity measurements, and Born–Oppenheimer molecular dynamic simulations.
中文翻译:

通过解决质子的作用了解氢氧化锂(Li2OHCl)固态电解质中的锂离子动力学
低熔点固态电解质(SSE)对于低成本制造全固态电池至关重要。羟基氯化锂(Li 2 OHCl)由于其低熔点(mp <300°C),廉价的成分(Li,H,O和Cl)和快速合成而成为SSE域中有希望的材料。该化合物的另一个独特特征是存在锂空位和旋转的羟基,它们促进锂离子的扩散,但对质子在离子迁移中的作用仍然知之甚少。为了检查锂和质子的动力学,我们进行了一组固态NMR实验,例如魔角旋转7 Li NMR,静态7 Li和1 H NMR和自旋晶格T 1(7 Li)/ T 1(1 H)弛豫实验。已确定只有Li +有助于长距离离子迁移,而H +动力学受限于OH基团的各向同性旋转不完全。结果揭示了Li 2 OHCl中离子迁移的详细机理。结果表明,在低温和高温下会出现两个明显的离子运动相,并且OH基团的旋转控制了两个相中的Li +和H +动力学。基于NMR实验的模型与晶体学信息,离子电导率测量以及Born–Oppenheimer分子动力学模拟完全一致。
更新日期:2020-02-25
中文翻译:

通过解决质子的作用了解氢氧化锂(Li2OHCl)固态电解质中的锂离子动力学
低熔点固态电解质(SSE)对于低成本制造全固态电池至关重要。羟基氯化锂(Li 2 OHCl)由于其低熔点(mp <300°C),廉价的成分(Li,H,O和Cl)和快速合成而成为SSE域中有希望的材料。该化合物的另一个独特特征是存在锂空位和旋转的羟基,它们促进锂离子的扩散,但对质子在离子迁移中的作用仍然知之甚少。为了检查锂和质子的动力学,我们进行了一组固态NMR实验,例如魔角旋转7 Li NMR,静态7 Li和1 H NMR和自旋晶格T 1(7 Li)/ T 1(1 H)弛豫实验。已确定只有Li +有助于长距离离子迁移,而H +动力学受限于OH基团的各向同性旋转不完全。结果揭示了Li 2 OHCl中离子迁移的详细机理。结果表明,在低温和高温下会出现两个明显的离子运动相,并且OH基团的旋转控制了两个相中的Li +和H +动力学。基于NMR实验的模型与晶体学信息,离子电导率测量以及Born–Oppenheimer分子动力学模拟完全一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号