当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of aryl 2,2,2-trifluoroethyl sulfides
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.jfluchem.2020.109464 Bálint Menczinger , Anikó Nemes , Dénes Szabó , Gitta Schlosser , Tamás Jernei , Antal Csámpai , József Rábai
中文翻译:

芳基2,2,2-三氟乙基硫醚的合成
更新日期:2020-01-17
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.jfluchem.2020.109464 Bálint Menczinger , Anikó Nemes , Dénes Szabó , Gitta Schlosser , Tamás Jernei , Antal Csámpai , József Rábai
|
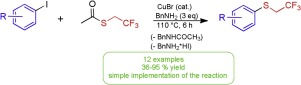
Aryl 2,2,2-trifluoroethyl sulfides were synthesized by copper(I)-catalyzed nucleophilic aromatic substitution reaction (Ullmann coupling). The method requires aryl iodides and 2,2,2-trifluoroethyl thioacetate as starting materials, benzylamine as solvent and base, and a copper(I) bromide as a catalyst. The reaction mixture was stirred at 110 °C for 6 h under inert atmosphere to afford the targeted aryl 2,2,2-trifluoroethyl sulfides in moderate to good yield.
中文翻译:

芳基2,2,2-三氟乙基硫醚的合成
通过铜(I)催化的亲核芳族取代反应(Ullmann偶联)合成了芳基2,2,2-三氟乙基硫化物。该方法需要芳基碘化物和2,2,2-三氟乙基硫代乙酸酯作为起始原料,苄胺作为溶剂和碱,以及溴化铜(I)作为催化剂。将反应混合物在惰性气氛下在110℃搅拌6h,以中等至良好的产率得到目标芳基2,2,2-三氟乙基硫醚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号