当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted camptothecin delivery via silicon nanoparticles reduces breast cancer metastasis.
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.biomaterials.2020.119791 Marietta Landgraf 1 , Christoph A Lahr 1 , Ishdeep Kaur 2 , Abbas Shafiee 3 , Alvaro Sanchez-Herrero 1 , Phillip W Janowicz 4 , Akhilandeshwari Ravichandran 1 , Christopher B Howard 5 , Anna Cifuentes-Rius 2 , Jacqui A McGovern 1 , Nicolas H Voelcker 6 , Dietmar W Hutmacher 1
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-17 , DOI: 10.1016/j.biomaterials.2020.119791 Marietta Landgraf 1 , Christoph A Lahr 1 , Ishdeep Kaur 2 , Abbas Shafiee 3 , Alvaro Sanchez-Herrero 1 , Phillip W Janowicz 4 , Akhilandeshwari Ravichandran 1 , Christopher B Howard 5 , Anna Cifuentes-Rius 2 , Jacqui A McGovern 1 , Nicolas H Voelcker 6 , Dietmar W Hutmacher 1
Affiliation
|
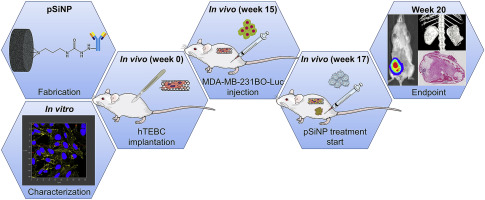
In advanced breast cancer (BCa) patients, not the primary tumor, but the development of distant metastases, which occur mainly in the organ bone, and their adverse health effects are responsible for high mortality. Targeted delivery of already known drugs which displayed potency, but rather unfavorable pharmacokinetic properties, might be a promising approach to overcome the current limitations of metastatic BCa therapy. Camptothecin (CPT) is a highly cytotoxic chemotherapeutic compound, yet poorly water-soluble and non-specific. Here, CPT was loaded into porous silicon nanoparticles (pSiNP) displaying the epidermal growth factor receptor (EGFR)-targeting antibody (Ab) cetuximab to generate a soluble and targeted nanoscale delivery vehicle for cancer treatment. After confirming the cytotoxic effect of targeted CPT-loaded pSiNP in vitro on MDA-MB-231BO cells, nanoparticles were studied in a humanized BCa bone metastasis mouse model. Humanized tissue-engineered bone constructs (hTEBCs) provided a humanized microenvironment for BCa bone metastases in female NOD-scid IL2Rgnull (NSG) mice. Actively targeted CPT-loaded pSiNP led to a reduction of orthotopic primary tumor growth, increased survival rate and significant decrease in hTEBC and murine lung, liver and bone metastases. This study demonstrates that targeted delivery via pSiNP is an effective approach to employ CPT and other potent anti-cancer compounds with poor pharmacokinetic profiles in cancer therapy.
中文翻译:

通过硅纳米颗粒靶向喜树碱的递送减少了乳腺癌的转移。
在晚期乳腺癌(BCa)患者中,不是原发性肿瘤,而是远处转移的发展,主要发生在器官骨骼中,其不良的健康影响导致高死亡率。有针对性地递送显示出效力但相对不利的药代动力学特性的已知药物,可能是克服转移性BCa治疗当前局限性的有前途的方法。喜树碱(CPT)是一种具有高度细胞毒性的化学治疗化合物,但水溶性差且非特异性。在此,将CPT加载到具有表皮生长因子受体(EGFR)靶向抗体(Ab)西妥昔单抗的多孔硅纳米颗粒(pSiNP)中,以生成可溶的靶向纳米级癌症治疗载体。在确认靶向CPT加载的pSiNP在体外对MDA-MB-231BO细胞的细胞毒性作用后,在人源化BCa骨转移小鼠模型中研究了纳米颗粒。人源化的组织工程化骨构建体(hTEBC)为雌性NOD-scid IL2Rgnull(NSG)小鼠的BCa骨转移提供了人源化的微环境。积极靶向加载CPT的pSiNP导致原位原发肿瘤生长减少,存活率提高以及hTEBC和鼠肺,肝和骨转移明显减少。这项研究表明,通过pSiNP靶向递送是在癌症治疗中采用CPT和其他药物动力学较差的有效抗癌化合物的有效方法。人源化的组织工程化骨构建体(hTEBC)为雌性NOD-scid IL2Rgnull(NSG)小鼠的BCa骨转移提供了人源化的微环境。积极靶向加载CPT的pSiNP导致原位原发肿瘤生长减少,存活率提高以及hTEBC和鼠肺,肝和骨转移明显减少。这项研究表明,通过pSiNP靶向递送是在癌症治疗中采用CPT和其他药物动力学较差的有效抗癌化合物的有效方法。人源化的组织工程化骨构建体(hTEBC)为雌性NOD-scid IL2Rgnull(NSG)小鼠的BCa骨转移提供了人源化的微环境。积极靶向加载CPT的pSiNP导致原位原发肿瘤生长减少,存活率提高以及hTEBC和鼠肺,肝和骨转移明显减少。这项研究表明,通过pSiNP靶向递送是在癌症治疗中采用CPT和其他药物动力学较差的有效抗癌化合物的有效方法。
更新日期:2020-01-17
中文翻译:

通过硅纳米颗粒靶向喜树碱的递送减少了乳腺癌的转移。
在晚期乳腺癌(BCa)患者中,不是原发性肿瘤,而是远处转移的发展,主要发生在器官骨骼中,其不良的健康影响导致高死亡率。有针对性地递送显示出效力但相对不利的药代动力学特性的已知药物,可能是克服转移性BCa治疗当前局限性的有前途的方法。喜树碱(CPT)是一种具有高度细胞毒性的化学治疗化合物,但水溶性差且非特异性。在此,将CPT加载到具有表皮生长因子受体(EGFR)靶向抗体(Ab)西妥昔单抗的多孔硅纳米颗粒(pSiNP)中,以生成可溶的靶向纳米级癌症治疗载体。在确认靶向CPT加载的pSiNP在体外对MDA-MB-231BO细胞的细胞毒性作用后,在人源化BCa骨转移小鼠模型中研究了纳米颗粒。人源化的组织工程化骨构建体(hTEBC)为雌性NOD-scid IL2Rgnull(NSG)小鼠的BCa骨转移提供了人源化的微环境。积极靶向加载CPT的pSiNP导致原位原发肿瘤生长减少,存活率提高以及hTEBC和鼠肺,肝和骨转移明显减少。这项研究表明,通过pSiNP靶向递送是在癌症治疗中采用CPT和其他药物动力学较差的有效抗癌化合物的有效方法。人源化的组织工程化骨构建体(hTEBC)为雌性NOD-scid IL2Rgnull(NSG)小鼠的BCa骨转移提供了人源化的微环境。积极靶向加载CPT的pSiNP导致原位原发肿瘤生长减少,存活率提高以及hTEBC和鼠肺,肝和骨转移明显减少。这项研究表明,通过pSiNP靶向递送是在癌症治疗中采用CPT和其他药物动力学较差的有效抗癌化合物的有效方法。人源化的组织工程化骨构建体(hTEBC)为雌性NOD-scid IL2Rgnull(NSG)小鼠的BCa骨转移提供了人源化的微环境。积极靶向加载CPT的pSiNP导致原位原发肿瘤生长减少,存活率提高以及hTEBC和鼠肺,肝和骨转移明显减少。这项研究表明,通过pSiNP靶向递送是在癌症治疗中采用CPT和其他药物动力学较差的有效抗癌化合物的有效方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号