当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering ApoE3-incorporated biomimetic nanoparticle for efficient vaccine delivery to dendritic cells via macropinocytosis to enhance cancer immunotherapy.
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.biomaterials.2020.119795 Songlei Zhou 1 , Yukun Huang 1 , Yu Chen 1 , Shanshan Liu 1 , Minjun Xu 1 , Tianze Jiang 1 , Qingxiang Song 2 , Gan Jiang 2 , Xiao Gu 2 , Xiaoling Gao 2 , Jun Chen 1
Biomaterials ( IF 12.8 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.biomaterials.2020.119795 Songlei Zhou 1 , Yukun Huang 1 , Yu Chen 1 , Shanshan Liu 1 , Minjun Xu 1 , Tianze Jiang 1 , Qingxiang Song 2 , Gan Jiang 2 , Xiao Gu 2 , Xiaoling Gao 2 , Jun Chen 1
Affiliation
|
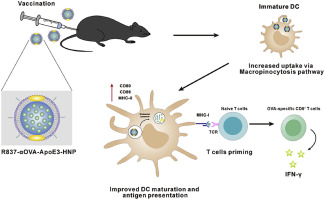
Efficient delivery of vaccines to dendritic cells (DCs) is critical for inducing sufficient immune response and realizing effective cancer immunotherapy. In the past decade, researchers have spent tremendous effort in delivering vaccines by using nanoparticles. However, most of the present strategies are designed based on receptor-mediated endocytosis to increase nanovaccines uptake by DCs, and underestimate the role of macropinocytosis in taking up exogenous antigen. Here, we proposed that macropinocytosis, an efficient pathway for DCs to internalize extracellular fluid-phase solutes, might be utilized as a highly-effective approach to facilitate nanovaccines uptake in DCs. Accordingly, we designed a biomimetic nanovaccine (R837-αOVA-ApoE3-HNP), composing of a poly-(D, l-lactide-co-glycolide) (PLGA) core to encapsulate adjuvant imiquimod (R837), a phospholipid membrane to load antigen peptide (αOVA), and apolipoprotein E3 (ApoE3), to boost the internalization of antigens into DCs. The nanovaccine exhibited highly efficient cellular uptake into DCs through the macropinocytosis pathway, and significantly promoted DCs maturation and antigen presentation. After subcutaneous injection, the nanovaccine was efficiently drained to lymph nodes. Strong T cell immune responses including the generation of antigen-specific CD8+ T cells, expansion of IFN-γ+ CD8+ T cells and the secretion of IFN-γ+ were observed after the vaccination of R837-αOVA-ApoE3-HNP. It also efficiently inhibited the formation of tumor metastasis in lung as a prevention vaccine, and exerted superior therapeutic efficiency on B16-OVA tumor-bearing mice when in combination with αPD-1 therapy. Overall, our work demonstrated that by utilizing the macropinocytosis pathway, ApoE3-incorporated biomimetic nanoparticle has great potential to function as a feasible, effective, and safe nanovaccine for cancer immunotherapy.
中文翻译:

工程化整合了ApoE3的仿生纳米颗粒,可通过巨胞饮作用将疫苗有效地递送至树突状细胞,从而增强癌症免疫疗法。
疫苗向树突状细胞(DC)的有效递送对于诱导足够的免疫应答和实现有效的癌症免疫疗法至关重要。在过去的十年中,研究人员在使用纳米颗粒运送疫苗方面付出了巨大的努力。然而,目前大多数策略是基于受体介导的内吞作用来设计的,以增加DC对纳米疫苗的吸收,并低估了巨胞吞作用在吸收外源抗原中的作用。在这里,我们建议巨胞饮作用,DC内在化细胞外液相溶质的DC的有效途径,可以用作促进DC中的纳米疫苗吸收的高效方法。因此,我们设计了一种仿生纳米疫苗(R837-αOVA-ApoE3-HNP),由聚(D,l-丙交酯-乙交酯)(PLGA)核组成,用于封装辅助咪喹莫特(R837),磷脂膜可加载抗原肽(αOVA)和载脂蛋白E3(ApoE3),以增强抗原向DC的内在化。纳米疫苗通过巨胞饮途径表现出对DC的高效细胞摄取,并显着促进DC的成熟和抗原呈递。皮下注射后,纳米疫苗被有效地引流至淋巴结。接种R837-αOVA-ApoE3-HNP疫苗后,观察到强T细胞免疫应答,包括抗原特异性CD8 + T细胞的产生,IFN-γ+ CD8 + T细胞的扩增和IFN-γ+的分泌。作为预防疫苗,它还可以有效地抑制肺中肿瘤转移的形成,并且与αPD-1治疗联合使用时,可以对B16-OVA荷瘤小鼠产生更高的治疗效果。总体,
更新日期:2020-01-17
中文翻译:

工程化整合了ApoE3的仿生纳米颗粒,可通过巨胞饮作用将疫苗有效地递送至树突状细胞,从而增强癌症免疫疗法。
疫苗向树突状细胞(DC)的有效递送对于诱导足够的免疫应答和实现有效的癌症免疫疗法至关重要。在过去的十年中,研究人员在使用纳米颗粒运送疫苗方面付出了巨大的努力。然而,目前大多数策略是基于受体介导的内吞作用来设计的,以增加DC对纳米疫苗的吸收,并低估了巨胞吞作用在吸收外源抗原中的作用。在这里,我们建议巨胞饮作用,DC内在化细胞外液相溶质的DC的有效途径,可以用作促进DC中的纳米疫苗吸收的高效方法。因此,我们设计了一种仿生纳米疫苗(R837-αOVA-ApoE3-HNP),由聚(D,l-丙交酯-乙交酯)(PLGA)核组成,用于封装辅助咪喹莫特(R837),磷脂膜可加载抗原肽(αOVA)和载脂蛋白E3(ApoE3),以增强抗原向DC的内在化。纳米疫苗通过巨胞饮途径表现出对DC的高效细胞摄取,并显着促进DC的成熟和抗原呈递。皮下注射后,纳米疫苗被有效地引流至淋巴结。接种R837-αOVA-ApoE3-HNP疫苗后,观察到强T细胞免疫应答,包括抗原特异性CD8 + T细胞的产生,IFN-γ+ CD8 + T细胞的扩增和IFN-γ+的分泌。作为预防疫苗,它还可以有效地抑制肺中肿瘤转移的形成,并且与αPD-1治疗联合使用时,可以对B16-OVA荷瘤小鼠产生更高的治疗效果。总体,










































 京公网安备 11010802027423号
京公网安备 11010802027423号