当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genomic programming of IRF4-expressing human Langerhans cells.
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-16 , DOI: 10.1038/s41467-019-14125-x Sofia Sirvent 1 , Andres F Vallejo 1 , James Davies 1 , Kalum Clayton 1 , Zhiguo Wu 2 , Jeongmin Woo 3 , Jeremy Riddell 4 , Virendra K Chaudhri 2, 5 , Patrick Stumpf 6 , Liliya Angelova Nazlamova 1 , Gabrielle Wheway 6 , Matthew Rose-Zerilli 7 , Jonathan West 7, 8 , Mario Pujato 9 , Xiaoting Chen 4 , Christopher H Woelk 10 , Ben MacArthur 7, 8 , Michael Ardern-Jones 1 , Peter S Friedmann 1 , Matthew T Weirauch 4, 11 , Harinder Singh 2, 5, 11 , Marta E Polak 1, 8
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-16 , DOI: 10.1038/s41467-019-14125-x Sofia Sirvent 1 , Andres F Vallejo 1 , James Davies 1 , Kalum Clayton 1 , Zhiguo Wu 2 , Jeongmin Woo 3 , Jeremy Riddell 4 , Virendra K Chaudhri 2, 5 , Patrick Stumpf 6 , Liliya Angelova Nazlamova 1 , Gabrielle Wheway 6 , Matthew Rose-Zerilli 7 , Jonathan West 7, 8 , Mario Pujato 9 , Xiaoting Chen 4 , Christopher H Woelk 10 , Ben MacArthur 7, 8 , Michael Ardern-Jones 1 , Peter S Friedmann 1 , Matthew T Weirauch 4, 11 , Harinder Singh 2, 5, 11 , Marta E Polak 1, 8
Affiliation
|
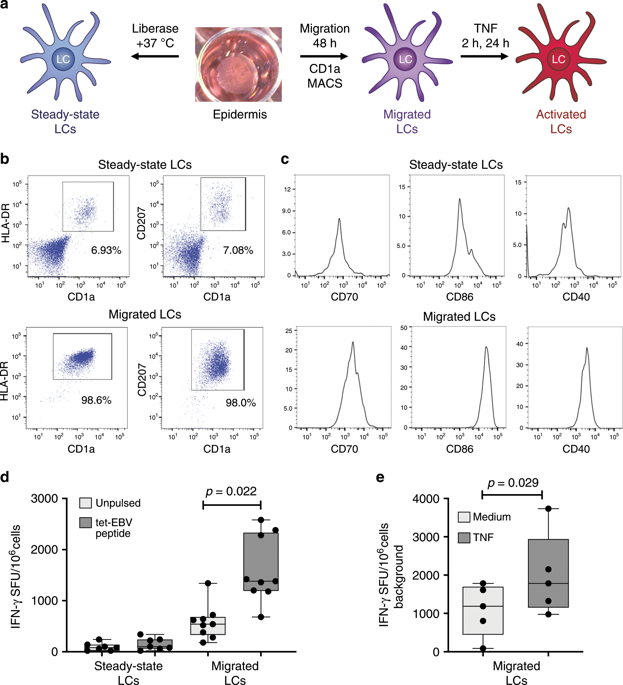
Langerhans cells (LC) can prime tolerogenic as well as immunogenic responses in skin, but the genomic states and transcription factors (TF) regulating these context-specific responses are unclear. Bulk and single-cell transcriptional profiling demonstrates that human migratory LCs are robustly programmed for MHC-I and MHC-II antigen presentation. Chromatin analysis reveals enrichment of ETS-IRF and AP1-IRF composite regulatory elements in antigen-presentation genes, coinciding with expression of the TFs, PU.1, IRF4 and BATF3 but not IRF8. Migration of LCs from the epidermis is accompanied by upregulation of IRF4, antigen processing components and co-stimulatory molecules. TNF stimulation augments LC cross-presentation while attenuating IRF4 expression. CRISPR-mediated editing reveals IRF4 to positively regulate the LC activation programme, but repress NF2EL2 and NF-kB pathway genes that promote responsiveness to oxidative stress and inflammatory cytokines. Thus, IRF4-dependent genomic programming of human migratory LCs appears to enable LC maturation while attenuating excessive inflammatory and immunogenic responses in the epidermis.
中文翻译:

表达IRF4的人Langerhans细胞的基因组编程。
朗格汉斯细胞(LC)可以引发皮肤的致耐受性和免疫原性应答,但是调节这些背景特异性应答的基因组状态和转录因子(TF)尚不清楚。批量和单细胞转录谱分析表明,人类迁徙LCs被强健地编程为MHC-I和MHC-II抗原呈递。染色质分析揭示了抗原呈递基因中ETS-IRF和AP1-IRF复合调控元件的富集,与TF,PU.1,IRF4和BATF3而不是IRF8的表达相吻合。LC从表皮的迁移伴随着IRF4,抗原加工成分和共刺激分子的上调。TNF刺激可增强LC交叉表达,同时减弱IRF4表达。CRISPR介导的编辑显示IRF4可以积极调节LC激活程序,但抑制NF2EL2和NF-kB通路基因,这些基因可促进对氧化应激和炎性细胞因子的反应。因此,人类迁移LC的依赖IRF4的基因组编程似乎能够使LC成熟,同时减轻表皮中过度的炎症和免疫原性反应。
更新日期:2020-01-16
中文翻译:

表达IRF4的人Langerhans细胞的基因组编程。
朗格汉斯细胞(LC)可以引发皮肤的致耐受性和免疫原性应答,但是调节这些背景特异性应答的基因组状态和转录因子(TF)尚不清楚。批量和单细胞转录谱分析表明,人类迁徙LCs被强健地编程为MHC-I和MHC-II抗原呈递。染色质分析揭示了抗原呈递基因中ETS-IRF和AP1-IRF复合调控元件的富集,与TF,PU.1,IRF4和BATF3而不是IRF8的表达相吻合。LC从表皮的迁移伴随着IRF4,抗原加工成分和共刺激分子的上调。TNF刺激可增强LC交叉表达,同时减弱IRF4表达。CRISPR介导的编辑显示IRF4可以积极调节LC激活程序,但抑制NF2EL2和NF-kB通路基因,这些基因可促进对氧化应激和炎性细胞因子的反应。因此,人类迁移LC的依赖IRF4的基因组编程似乎能够使LC成熟,同时减轻表皮中过度的炎症和免疫原性反应。







































 京公网安备 11010802027423号
京公网安备 11010802027423号