当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Maximizing the ovarian reserve in mice by evading LINE-1 genotoxicity.
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-16 , DOI: 10.1038/s41467-019-14055-8 Marla E Tharp 1, 2 , Safia Malki 1 , Alex Bortvin 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-16 , DOI: 10.1038/s41467-019-14055-8 Marla E Tharp 1, 2 , Safia Malki 1 , Alex Bortvin 1
Affiliation
|
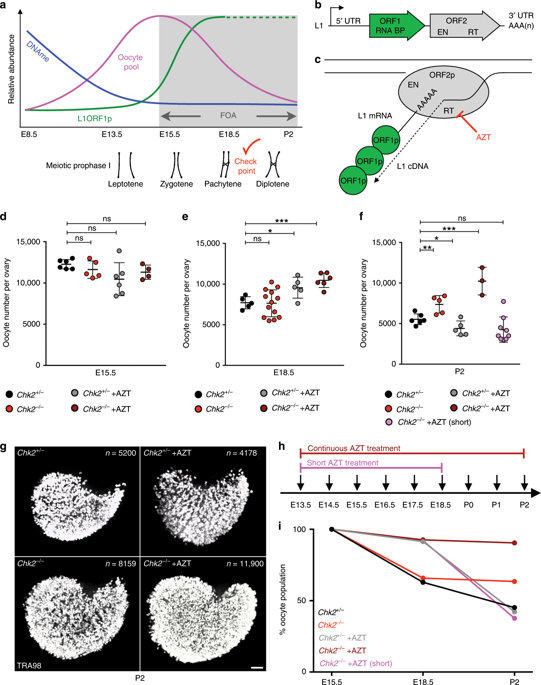
Female reproductive success critically depends on the size and quality of a finite ovarian reserve. Paradoxically, mammals eliminate up to 80% of the initial oocyte pool through the enigmatic process of fetal oocyte attrition (FOA). Here, we interrogate the striking correlation of FOA with retrotransposon LINE-1 (L1) expression in mice to understand how L1 activity influences FOA and its biological relevance. We report that L1 activity triggers FOA through DNA damage-driven apoptosis and the complement system of immunity. We demonstrate this by combined inhibition of L1 reverse transcriptase activity and the Chk2-dependent DNA damage checkpoint to prevent FOA. Remarkably, reverse transcriptase inhibitor AZT-treated Chk2 mutant oocytes that evade FOA initially accumulate, but subsequently resolve, L1-instigated genotoxic threats independent of piRNAs and differentiate, resulting in an increased functional ovarian reserve. We conclude that FOA serves as quality control for oocyte genome integrity, and is not obligatory for oogenesis nor fertility.
中文翻译:

通过逃避 LINE-1 基因毒性来最大化小鼠的卵巢储备。
女性生殖成功很大程度上取决于有限卵巢储备的大小和质量。矛盾的是,哺乳动物通过胎儿卵母细胞损耗 (FOA) 的神秘过程消除了高达 80% 的初始卵母细胞池。在这里,我们探讨了小鼠体内 FOA 与逆转录转座子 LINE-1 (L1) 表达的显着相关性,以了解 L1 活性如何影响 FOA 及其生物学相关性。我们报告,L1 活性通过 DNA 损伤驱动的细胞凋亡和免疫补体系统触发 FOA。我们通过联合抑制 L1 逆转录酶活性和 Chk2 依赖性 DNA 损伤检查点来预防 FOA 来证明这一点。值得注意的是,逆转录酶抑制剂 AZT 处理的 Chk2 突变卵母细胞最初会积累,但随后会独立于 piRNA 解决 L1 引发的基因毒性威胁并分化,从而导致卵巢储备功能增加。我们得出的结论是,FOA 可作为卵母细胞基因组完整性的质量控制,并且对于卵子发生或生育力不是必需的。
更新日期:2020-01-16
中文翻译:

通过逃避 LINE-1 基因毒性来最大化小鼠的卵巢储备。
女性生殖成功很大程度上取决于有限卵巢储备的大小和质量。矛盾的是,哺乳动物通过胎儿卵母细胞损耗 (FOA) 的神秘过程消除了高达 80% 的初始卵母细胞池。在这里,我们探讨了小鼠体内 FOA 与逆转录转座子 LINE-1 (L1) 表达的显着相关性,以了解 L1 活性如何影响 FOA 及其生物学相关性。我们报告,L1 活性通过 DNA 损伤驱动的细胞凋亡和免疫补体系统触发 FOA。我们通过联合抑制 L1 逆转录酶活性和 Chk2 依赖性 DNA 损伤检查点来预防 FOA 来证明这一点。值得注意的是,逆转录酶抑制剂 AZT 处理的 Chk2 突变卵母细胞最初会积累,但随后会独立于 piRNA 解决 L1 引发的基因毒性威胁并分化,从而导致卵巢储备功能增加。我们得出的结论是,FOA 可作为卵母细胞基因组完整性的质量控制,并且对于卵子发生或生育力不是必需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号