当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiphase Chemistry Controls Inorganic Chlorinated and Nitrogenated Compounds in Indoor Air during Bleach Cleaning.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.est.9b05767 James M Mattila 1 , Pascale S J Lakey 2 , Manabu Shiraiwa 2 , Chen Wang 3 , Jonathan P D Abbatt 3 , Caleb Arata 4, 5 , Allen H Goldstein 5, 6 , Laura Ampollini 7 , Erin F Katz 8 , Peter F DeCarlo 7, 8 , Shan Zhou 9 , Tara F Kahan 9, 10 , Felipe J Cardoso-Saldaña 11 , Lea Hildebrandt Ruiz 11 , Andrew Abeleira 1 , Erin K Boedicker 1 , Marina E Vance 12 , Delphine K Farmer 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.est.9b05767 James M Mattila 1 , Pascale S J Lakey 2 , Manabu Shiraiwa 2 , Chen Wang 3 , Jonathan P D Abbatt 3 , Caleb Arata 4, 5 , Allen H Goldstein 5, 6 , Laura Ampollini 7 , Erin F Katz 8 , Peter F DeCarlo 7, 8 , Shan Zhou 9 , Tara F Kahan 9, 10 , Felipe J Cardoso-Saldaña 11 , Lea Hildebrandt Ruiz 11 , Andrew Abeleira 1 , Erin K Boedicker 1 , Marina E Vance 12 , Delphine K Farmer 1
Affiliation
|
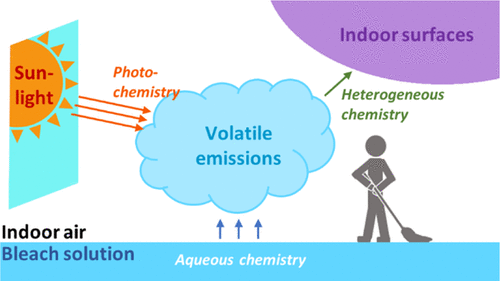
We report elevated levels of gaseous inorganic chlorinated and nitrogenated compounds in indoor air while cleaning with a commercial bleach solution during the House Observations of Microbial and Environmental Chemistry field campaign in summer 2018. Hypochlorous acid (HOCl), chlorine (Cl2), and nitryl chloride (ClNO2) reached part-per-billion by volume levels indoors during bleach cleaning-several orders of magnitude higher than typically measured in the outdoor atmosphere. Kinetic modeling revealed that multiphase chemistry plays a central role in controlling indoor chlorine and reactive nitrogen chemistry during these periods. Cl2 production occurred via heterogeneous reactions of HOCl on indoor surfaces. ClNO2 and chloramine (NH2Cl, NHCl2, NCl3) production occurred in the applied bleach via aqueous reactions involving nitrite (NO2-) and ammonia (NH3), respectively. Aqueous-phase and surface chemistry resulted in elevated levels of gas-phase nitrogen dioxide (NO2). We predict hydroxyl (OH) and chlorine (Cl) radical production during these periods (106 and 107 molecules cm-3 s-1, respectively) driven by HOCl and Cl2 photolysis. Ventilation and photolysis accounted for <50% and <0.1% total loss of bleach-related compounds from indoor air, respectively; we conclude that uptake to indoor surfaces is an important additional loss process. Indoor HOCl and nitrogen trichloride (NCl3) mixing ratios during bleach cleaning reported herein are likely detrimental to human health.
中文翻译:

在漂白过程中,多相化学可控制室内空气中的无机氯化和氮化合物。
我们在2018年夏季的“众议院微生物与环境化学观察”野外活动期间报告了在室内空气中气态无机氯化物和氯化物含量升高的同时,用商业漂白剂溶液进行清洁的情况。次氯酸(HOCl),氯(Cl2)和丁酰氯在漂白剂清洗过程中,室内的(ClNO2)含量达到了十亿分之几的水平,比室外大气中通常测量的数量级高出几个数量级。动力学建模表明,在这些时期中,多相化学在控制室内氯和反应性氮化学中起着核心作用。Cl2的产生是通过HOCl在室内表面上的异质反应而发生的。ClNO2和氯胺(NH2Cl,NHCl2,通过分别涉及亚硝酸盐(NO2-)和氨水(NH3)的水性反应,在所施加的漂白剂中产生了NCl3)。水相和表面化学作用导致气相二氧化氮(NO2)含量升高。我们预测由HOCl和Cl2光解驱动的这两个时期(分别为106和107分子cm-3 s-1)产生的羟基(OH)和氯(Cl)自由基。通风和光解分别占室内空气中与漂白剂有关的化合物总损失的<50%和<0.1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。水相和表面化学作用导致气相二氧化氮(NO2)含量升高。我们预测由HOCl和Cl2光解驱动的这两个时期(分别为106和107分子cm-3 s-1)产生的羟基(OH)和氯(Cl)自由基。通风和光解分别占室内空气中与漂白剂有关的化合物总损失的<50%和<0.1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。水相和表面化学作用导致气相二氧化氮(NO2)含量升高。我们预测由HOCl和Cl2光解驱动的这两个时期(分别为106和107分子cm-3 s-1)产生的羟基(OH)和氯(Cl)自由基。通风和光解分别占室内空气中与漂白剂有关的化合物总损失的<50%和<0.1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。室内空气中与漂白剂有关的化合物的总损失分别为1%;我们得出结论,摄入室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。室内空气中与漂白剂有关的化合物的总损失分别为1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。
更新日期:2020-01-26
中文翻译:

在漂白过程中,多相化学可控制室内空气中的无机氯化和氮化合物。
我们在2018年夏季的“众议院微生物与环境化学观察”野外活动期间报告了在室内空气中气态无机氯化物和氯化物含量升高的同时,用商业漂白剂溶液进行清洁的情况。次氯酸(HOCl),氯(Cl2)和丁酰氯在漂白剂清洗过程中,室内的(ClNO2)含量达到了十亿分之几的水平,比室外大气中通常测量的数量级高出几个数量级。动力学建模表明,在这些时期中,多相化学在控制室内氯和反应性氮化学中起着核心作用。Cl2的产生是通过HOCl在室内表面上的异质反应而发生的。ClNO2和氯胺(NH2Cl,NHCl2,通过分别涉及亚硝酸盐(NO2-)和氨水(NH3)的水性反应,在所施加的漂白剂中产生了NCl3)。水相和表面化学作用导致气相二氧化氮(NO2)含量升高。我们预测由HOCl和Cl2光解驱动的这两个时期(分别为106和107分子cm-3 s-1)产生的羟基(OH)和氯(Cl)自由基。通风和光解分别占室内空气中与漂白剂有关的化合物总损失的<50%和<0.1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。水相和表面化学作用导致气相二氧化氮(NO2)含量升高。我们预测由HOCl和Cl2光解驱动的这两个时期(分别为106和107分子cm-3 s-1)产生的羟基(OH)和氯(Cl)自由基。通风和光解分别占室内空气中与漂白剂有关的化合物总损失的<50%和<0.1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。水相和表面化学作用导致气相二氧化氮(NO2)含量升高。我们预测由HOCl和Cl2光解驱动的这两个时期(分别为106和107分子cm-3 s-1)产生的羟基(OH)和氯(Cl)自由基。通风和光解分别占室内空气中与漂白剂有关的化合物总损失的<50%和<0.1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。室内空气中与漂白剂有关的化合物的总损失分别为1%;我们得出结论,摄入室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。室内空气中与漂白剂有关的化合物的总损失分别为1%;我们得出结论,摄取到室内表面是重要的附加损失过程。本文报道的在漂白剂清洁期间的室内HOCl和三氯化氮(NCl 3)的混合比可能对人体健康有害。











































 京公网安备 11010802027423号
京公网安备 11010802027423号