当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coordination Structures of Uranium(VI) and Plutonium(IV) in Organic Solutions with Amide Derivatives.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.inorgchem.9b03024 Clémence Berger 1 , Cécile Marie 1 , Dominique Guillaumont 1 , Christelle Tamain 1 , Thomas Dumas 1 , Thomas Dirks 1 , Nathalie Boubals 1 , Eléonor Acher 1 , Marjorie Laszczyk 1 , Laurence Berthon 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.inorgchem.9b03024 Clémence Berger 1 , Cécile Marie 1 , Dominique Guillaumont 1 , Christelle Tamain 1 , Thomas Dumas 1 , Thomas Dirks 1 , Nathalie Boubals 1 , Eléonor Acher 1 , Marjorie Laszczyk 1 , Laurence Berthon 1
Affiliation
|
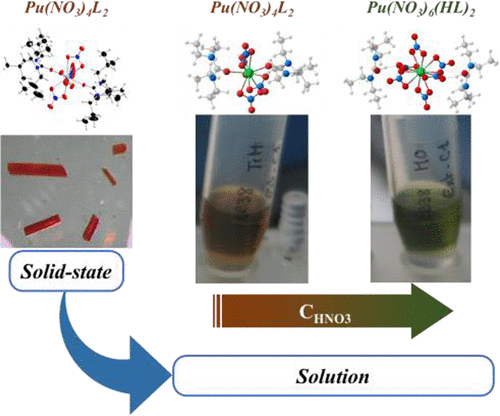
Carbamide and monoamide derivatives are very promising molecules to achieve U(VI) and Pu(IV) extraction and separation from spent nuclear fuels through solvent extraction. Herein, coordination structures of U(VI) and Pu(IV) complexes with carbamide derivatives were characterized using X-ray crystallography as well as infrared, UV-visible, and EXAFS spectroscopies. Coordination structures are compared to those obtained for monoamide derivatives in order to better understand the role of coordination chemistry in extraction properties. Single crystals were first synthesized with a short alkyl chain carbamide analog. Carbamide complexation in the solid state is found analogous to that in the monoamide. In organic solution, upon solvent extraction from nitric acid aqueous solution, it is shown that both amide derivatives can bind in the inner and outer coordination spheres of uranium(VI) and plutonium(IV). The amount of outer sphere coordination complexes increases with the amount of nitric acid. With uranium(VI), at a nitric acid concentration up to 5 mol·L-1, amide derivatives operate predominantly in the inner coordination sphere. In contrast, Pu(IV) coordination geometry is much more sensitive toward acid concentration or ligand structure than U(VI). Pu(IV) changes from inner sphere complexation at 0.5 mol·L-1 HNO3 to mostly outer sphere complexation at 4 mol·L-1. The proportion of outer-sphere complexes is strongly influenced by the ligand structure. Higher Pu(IV) extraction is found to be correlated with the amount of Pu(IV) outer sphere species. Secondary interactions in the outer sphere coordination shell appear to be of primary importance for plutonium extraction.
中文翻译:

含酰胺衍生物的有机溶液中铀(VI)和P(IV)的配位结构。
碳酰胺和单酰胺衍生物是实现U(VI)和Pu(IV)萃取以及通过溶剂萃取从乏核燃料中分离的非常有前途的分子。本文中,使用X射线晶体学以及红外光谱,紫外可见光谱和EXAFS光谱学表征了U(VI)和Pu(IV)配合物与尿素衍生物的配位结构。将配位结构与单酰胺衍生物获得的配位结构进行了比较,以更好地理解配位化学在萃取性质中的作用。首先用短烷基链脲类似物合成单晶。发现固态的氨基甲酸酯络合物与单酰胺中的类似。在有机溶液中,从硝酸水溶液中提取溶剂后,结果表明,两种酰胺衍生物都可以在铀(VI)和p(IV)的内部和外部配位球中结合。外球配位配合物的量随硝酸的量而增加。对于铀(VI),在硝酸浓度最高为5mol·L-1的情况下,酰胺衍生物主要在内部配位球中起作用。相反,Pu(IV)配位几何结构对酸浓度或配体结构的敏感性比U(VI)高得多。Pu(IV)从0.5mol·L-1HNO3的内球络合物变为4mol·L-1的外球络合物。外球配合物的比例受配体结构的强烈影响。发现较高的Pu(IV)提取量与Pu(IV)外球物种的数量有关。
更新日期:2020-01-16
中文翻译:

含酰胺衍生物的有机溶液中铀(VI)和P(IV)的配位结构。
碳酰胺和单酰胺衍生物是实现U(VI)和Pu(IV)萃取以及通过溶剂萃取从乏核燃料中分离的非常有前途的分子。本文中,使用X射线晶体学以及红外光谱,紫外可见光谱和EXAFS光谱学表征了U(VI)和Pu(IV)配合物与尿素衍生物的配位结构。将配位结构与单酰胺衍生物获得的配位结构进行了比较,以更好地理解配位化学在萃取性质中的作用。首先用短烷基链脲类似物合成单晶。发现固态的氨基甲酸酯络合物与单酰胺中的类似。在有机溶液中,从硝酸水溶液中提取溶剂后,结果表明,两种酰胺衍生物都可以在铀(VI)和p(IV)的内部和外部配位球中结合。外球配位配合物的量随硝酸的量而增加。对于铀(VI),在硝酸浓度最高为5mol·L-1的情况下,酰胺衍生物主要在内部配位球中起作用。相反,Pu(IV)配位几何结构对酸浓度或配体结构的敏感性比U(VI)高得多。Pu(IV)从0.5mol·L-1HNO3的内球络合物变为4mol·L-1的外球络合物。外球配合物的比例受配体结构的强烈影响。发现较高的Pu(IV)提取量与Pu(IV)外球物种的数量有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号