当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting the Thermodynamic Stability of Zirconium Radiotracers.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.inorgchem.9b03515 Jason P Holland 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.inorgchem.9b03515 Jason P Holland 1
Affiliation
|
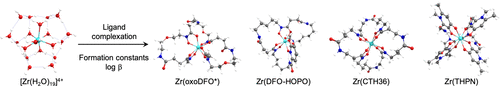
The thermodynamic stability of a metal-ligand complex, as measured by the formation constant (log β), is one of the most important parameters that determines metal ion selectivity and potential applications in, for example, radiopharmaceutical science. The stable coordination chemistry of radioactive 89Zr4+ in an aqueous environment is of paramount importance when developing positron-emitting radiotracers based on proteins (usually antibodies) for use with positron emission tomography. Desferrioxamine B (DFO) remains the chelate of choice for clinical applications of 89Zr-labeled proteins, but the coordination of DFO to Zr4+ ions is suboptimal. Many alternative ligands have been reported, but the challenges in measuring very high log β values with metal ions such as Zr4+ that tend to hydrolyze mean that accurate thermodynamic data are scarce. In this work, density functional theory (DFT) calculations were used to predict the reaction energetics for metal ion complexation. Computed values of pseudoformation constants (log β') are correlated with experimental data and showed an excellent linear relationship (R2 = 0.97). The model was then used to estimate the absolute and relative formation constants of 23 different Zr4+ complexes using a total of 17 different ligands, including many of the alternative bifunctional chelates that have been reported recently for use in 89Zr4+ radiochemistry. In addition, detailed computational studies were performed on the geometric isomerism and hydration state of Zr-desferrioxamine. Collectively, the results offer new insights into Zr4+ coordination chemistry that will help guide the synthesis of future ligands. The computational model developed here is straightforward and reproducible and can be readily applied in the design of other metal coordination compounds.
中文翻译:

预测锆放射性示踪剂的热力学稳定性。
金属-配体配合物的热力学稳定性(通过形成常数(logβ)测量)是最重要的参数之一,它决定了金属离子的选择性和在例如放射性药物科学中的潜在应用。当开发基于蛋白质(通常是抗体)的正电子发射放射性示踪剂以用于正电子发射断层扫描时,在水性环境中稳定的放射性89Zr4 +配位化学至关重要。Desferrioxamine B(DFO)仍然是89Zr标记蛋白临床应用的首选螯合物,但DFO与Zr4 +离子的配位不理想。已经报道了许多替代的配体,但是用倾向于水解的金属离子(例如Zr4 +)测量非常高的logβ值所面临的挑战意味着缺乏准确的热力学数据。在这项工作中,使用密度泛函理论(DFT)计算来预测金属离子络合的反应能。伪形成常数的计算值(logβ')与实验数据相关,并显示出极好的线性关系(R2 = 0.97)。然后使用该模型通过总共17种不同的配体(包括最近报道用于89Zr4 +放射化学的许多双功能螯合剂)来估计23种不同Zr4 +复合物的绝对和相对形成常数。另外,对Zr-去铁胺的几何异构和水合状态进行了详细的计算研究。总的来说,这些结果为Zr4 +配位化学提供了新的见解,将有助于指导未来配体的合成。
更新日期:2020-01-16
中文翻译:

预测锆放射性示踪剂的热力学稳定性。
金属-配体配合物的热力学稳定性(通过形成常数(logβ)测量)是最重要的参数之一,它决定了金属离子的选择性和在例如放射性药物科学中的潜在应用。当开发基于蛋白质(通常是抗体)的正电子发射放射性示踪剂以用于正电子发射断层扫描时,在水性环境中稳定的放射性89Zr4 +配位化学至关重要。Desferrioxamine B(DFO)仍然是89Zr标记蛋白临床应用的首选螯合物,但DFO与Zr4 +离子的配位不理想。已经报道了许多替代的配体,但是用倾向于水解的金属离子(例如Zr4 +)测量非常高的logβ值所面临的挑战意味着缺乏准确的热力学数据。在这项工作中,使用密度泛函理论(DFT)计算来预测金属离子络合的反应能。伪形成常数的计算值(logβ')与实验数据相关,并显示出极好的线性关系(R2 = 0.97)。然后使用该模型通过总共17种不同的配体(包括最近报道用于89Zr4 +放射化学的许多双功能螯合剂)来估计23种不同Zr4 +复合物的绝对和相对形成常数。另外,对Zr-去铁胺的几何异构和水合状态进行了详细的计算研究。总的来说,这些结果为Zr4 +配位化学提供了新的见解,将有助于指导未来配体的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号