The Lancet Respiratory Medicine ( IF 38.7 ) Pub Date : 2020-01-15 , DOI: 10.1016/s2213-2600(19)30267-x Daniel S-W Tan , Natasha B Leighl , Gregory J Riely , James C-H Yang , Lecia V Sequist , Juergen Wolf , Takashi Seto , Enriqueta Felip , Santiago P Aix , Maud Jonnaert , Chun Pan , Eugene Y Tan , Jinnie Ko , Susan E Moody , Dong-Wan Kim
|
Background
Resistance to first-generation and second-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) is mediated by the emergence of the Thr790Met mutation in 50–60% of treated patients with non-small-cell lung cancer (NSCLC). We aimed to assess the safety and activity of nazartinib (EGF816), a third-generation EGFR TKI that selectively inhibits EGFR with Thr790Met or activating mutations (or both), while sparing wild-type EGFR, in patients with advanced EGFR-mutant NSCLC.
Methods
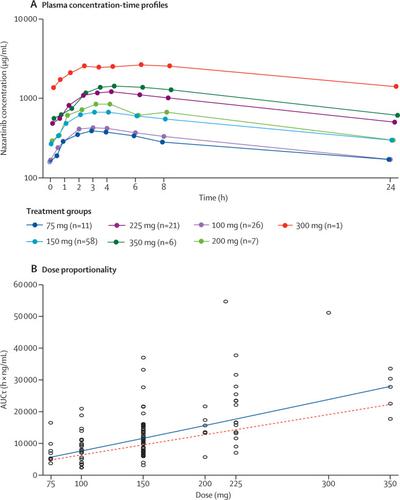
This phase 1 dose-escalation part of an open-label, multicentre, phase 1/2 study was conducted at nine academic medical centres located in Europe, Asia, and North America. Patients were included if they were aged 18 years or older and had stage IIIB–IV EGFR-mutant NSCLC (with varying statuses of EGFR mutation and previous therapy allowed), at least one measurable lesion, and an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less. Nazartinib (at seven dose levels between 75 mg and 350 mg, in capsule or tablet form) was administered orally, once daily, on a continuous 28-day dosing schedule. A two-parameter Bayesian logistic regression model, guided by the escalation with overdose control principle, was implemented to make dose recommendations and estimate the maximum tolerated dose or recommended phase 2 dose of nazartinib (the primary outcome). This study is registered with ClinicalTrials.gov (NCT02108964); enrolment to phase 1 is complete and the study is ongoing.
Findings
By Aug 31, 2017, 180 patients (116 [64%] women; median age 60 years (52–69); 116 [64%] with ECOG performance status 1) received nazartinib across seven dose levels: 75 mg (n=17), 100 mg (n=38), 150 mg (n=73), 200 mg (n=8), 225 mg (n=28), 300 mg (n=5), and 350 mg (n=11). Seven dose-limiting toxicities were observed in six (3%) patients who received 150 mg, 225 mg, or 350 mg nazartinib once daily. Although the maximum tolerated dose was not met, the recommended phase 2 dose was declared as 150 mg once daily (tablet). The most common adverse events, regardless of cause, were rash (all subcategories 111 [62%] patients, maculopapular rash 72 [40%], dermatitis acneiform 22 [12%]), diarrhoea (81 [45%]), pruritus (70 [39%]), fatigue (54 [30%]), and stomatitis (54 [30%]), and were mostly grades 1–2. Any-cause grade 3–4 adverse events were reported in 99 (55%) patients across all doses, the most common being rash (all subcategories grouped 27 [15%]), pneumonia (12 [7%]), anaemia (ten [6%]), and dyspnoea (nine [5%]). Serious adverse events suspected to be drug-related occurred in 16 (9%) patients.
Interpretation
Nazartinib has a favourable safety profile, with low-grade skin toxicity characterised by a predominantly maculopapular rash that required minimal dose reductions.
Funding
Novartis Pharmaceuticals Corporation.
中文翻译:

纳扎替尼(EGF816)在EGFR突变型非小细胞肺癌成人中的安全性和有效性:一项多中心,开放标签的1期研究。
背景
对第一代和第二代表皮生长因子受体(EGFR)酪氨酸激酶抑制剂(TKIs)的耐药性是由50-60%接受治疗的非小细胞肺癌(NSCLC)患者中的Thr790Met突变介导的。我们旨在评估晚期EGFR突变型NSCLC患者中第三代EGFR TKI纳扎替尼(EGF816)的安全性和活性,该抑制剂可选择性抑制具有Thr790Met或激活突变(或两者)的EGFR,同时保留野生型EGFR 。
方法
这项开放式,多中心,1/2期研究的1期剂量递增部分是在欧洲,亚洲和北美的9个学术医学中心进行的。如果患者年龄大于或等于18岁且患有IIIB–IV期EGFR突变NSCLC(EGFR的状态不同)突变和允许接受先前的治疗),至少一个可测量的病变以及东部合作肿瘤小组(ECOG)的表现状态为2以下。纳扎替尼(75毫克至350毫克的七个剂量水平,以胶囊或片剂形式)每天口服,连续28天给药。实施两参数贝叶斯逻辑回归模型,该模型以剂量控制原则逐步升级为指导,以提出剂量建议并估算纳扎替尼的最大耐受剂量或推荐的2期剂量(主要结局)。该研究已在ClinicalTrials.gov(NCT02108964)上注册;阶段1的注册已完成,研究正在进行中。
发现
截至2017年8月31日,接受纳扎替尼治疗的患者共有七个剂量水平:75毫克(n = 17)(180名患者(116名[64%];女性中位年龄60岁(52-69岁); 116名[64%] ECOG表现为1)。 ),100 mg(n = 38),150 mg(n = 73),200 mg(n = 8),225 mg(n = 28),300 mg(n = 5)和350 mg(n = 11) 。在每天接受150 mg,225 mg或350 mg纳扎替尼的六名(3%)患者中观察到七种剂量限制性毒性。尽管未达到最大耐受剂量,但宣布的推荐2期剂量为每天一次150毫克(片剂)。无论原因如何,最常见的不良事件为皮疹(所有子类别111 [62%]患者,斑丘疹72 [40%],痤疮性皮肤炎22 [12%]),腹泻(81 [45%]),瘙痒( 70 [39%]),疲劳(54 [30%])和口腔炎(54 [30%]),大多为1-2年级。在所有剂量下,有99名(55%)患者报告了任何原因的3-4级不良事件,最常见的是皮疹(所有亚类分组27 [15%]),肺炎(12 [7%]),贫血(十[6%])和呼吸困难(9 [5%])。怀疑与药物相关的严重不良事件发生在16(9%)位患者中。
解释
纳扎替尼具有良好的安全性,具有低度皮肤毒性,其特征是主要为斑丘疹,需要最小的剂量减少。
资金
诺华制药公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号