当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Phase Behavior of Solutions of 1,3-Diethylimidazolium Bis((trifluoromethyl)sulfonyl)amide in n-Alkyl Alcohols
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.jced.9b00800 Marisa A.A. Rocha 1 , Daniela Kerlé 1, 2 , Johannes Kiefer 1, 2 , Wolffram Schröer 3 , Bernd Rathke 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.jced.9b00800 Marisa A.A. Rocha 1 , Daniela Kerlé 1, 2 , Johannes Kiefer 1, 2 , Wolffram Schröer 3 , Bernd Rathke 1
Affiliation
|
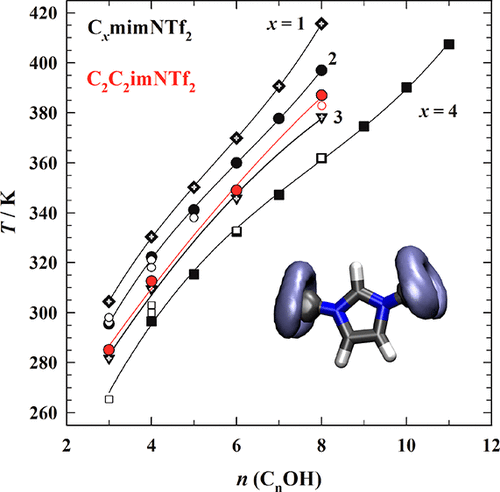
The cloud point method is applied for the characterization of the limited miscibility of ionic liquid (IL) solutions. Here, we report the phase behavior of binary mixtures of the ionic liquid 1,3-diethylimidazolium bis((trifluoromethyl)sulfonyl)amide (C2C2imNTf2) with n-alkyl alcohols (propan-1-ol, butan-1-ol, hexan-1-ol, and octan-1-ol) in a temperature range (T) of 258–386 K. The two components exhibit partial miscibility with upper critical solution temperatures (UCSTs) between 284 and 386 K. In concordance with previous studies, a detailed shape analysis presuming Ising behavior of the phase diagrams reveals the critical points and further properties of the phase diagrams. The results are compared with the isomeric IL C3mimNTf2 from the CxmimNTf2 family. Unraveling the influence of the molecular structure of the cation on the phase behavior is the main focus of the present investigation. The comparison between the two isomeric ILs, C2C2imNTf2 and C3mimNTf2, shows an increase of the UCST for the symmetric cation. A clear trend of lifting the UCST toward higher values is observed when dealing with longer chained n-alkyl alcohols. This behavior is put into context with a more generalized view on the phase behavior of the CxmimNTf2 IL family. An effective number of carbon atoms of the cationic side chains is determined for the mixtures with different alcohols. For alcohols with chains ≤6, the effective carbon number is constant and about 3.79, while for octan-1-ol, a slightly lower value of 3.55 was observed. That gives evidence of a change of the molecular ordering of the system. The experimental study is supported by molecular dynamics (MD) simulations to attain a better understanding at the molecular level. The local dynamics and structures are analyzed in detail and reveal the effect of the symmetry on different physicochemical properties.
中文翻译:

1,3-二乙基咪唑鎓双((三氟甲基)磺酰基)酰胺在正烷基醇中的溶液的液相行为
浊点法用于表征离子液体(IL)溶液的有限混溶性。在这里,我们报告离子液体1,3-二乙基咪唑双((三氟甲基)磺酰基)酰胺(C 2 C 2 imNTf 2)与正烷基醇(丙-1-醇,丁丹-1 )的二元混合物的相行为-ol,hexan-1-ol和octan-1-ol)在温度范围(T)在258–386 K之间。这两个成分在284至386 K的较高临界溶液温度(UCST)下表现出部分溶混性。与先前的研究一致,假定相图的Ising行为的详细形状分析揭示了临界点,并且进一步相图的属性。将结果与来自C x mimNTf 2家族的异构体IL C 3 mimNTf 2进行比较。揭示阳离子分子结构对相行为的影响是本研究的主要重点。C 2 C 2 imNTf 2和C 3 mimNTf 2这两种异构IL的比较,表明对称阳离子的UCST增加。当处理更长链的正烷基醇时,观察到明显的将UCST提升到更高值的趋势。将此行为与C x mimNTf 2的相位行为的更一般性的观点相结合IL家族。确定具有不同醇的混合物的阳离子侧链的有效碳原子数。对于链≤6的醇,有效碳数是恒定的,约为3.79,而对于octan-1-ol,观察到的碳数稍低,为3.55。这提供了系统分子顺序改变的证据。实验研究得到分子动力学(MD)模拟的支持,以便在分子水平上获得更好的理解。详细分析了局部动力学和结构,揭示了对称性对不同理化性质的影响。
更新日期:2020-01-16
中文翻译:

1,3-二乙基咪唑鎓双((三氟甲基)磺酰基)酰胺在正烷基醇中的溶液的液相行为
浊点法用于表征离子液体(IL)溶液的有限混溶性。在这里,我们报告离子液体1,3-二乙基咪唑双((三氟甲基)磺酰基)酰胺(C 2 C 2 imNTf 2)与正烷基醇(丙-1-醇,丁丹-1 )的二元混合物的相行为-ol,hexan-1-ol和octan-1-ol)在温度范围(T)在258–386 K之间。这两个成分在284至386 K的较高临界溶液温度(UCST)下表现出部分溶混性。与先前的研究一致,假定相图的Ising行为的详细形状分析揭示了临界点,并且进一步相图的属性。将结果与来自C x mimNTf 2家族的异构体IL C 3 mimNTf 2进行比较。揭示阳离子分子结构对相行为的影响是本研究的主要重点。C 2 C 2 imNTf 2和C 3 mimNTf 2这两种异构IL的比较,表明对称阳离子的UCST增加。当处理更长链的正烷基醇时,观察到明显的将UCST提升到更高值的趋势。将此行为与C x mimNTf 2的相位行为的更一般性的观点相结合IL家族。确定具有不同醇的混合物的阳离子侧链的有效碳原子数。对于链≤6的醇,有效碳数是恒定的,约为3.79,而对于octan-1-ol,观察到的碳数稍低,为3.55。这提供了系统分子顺序改变的证据。实验研究得到分子动力学(MD)模拟的支持,以便在分子水平上获得更好的理解。详细分析了局部动力学和结构,揭示了对称性对不同理化性质的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号