当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Validamycin Shunt Pathway for Valienamine Synthesis in Engineered Streptomyces hygroscopicus 5008.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-01-15 , DOI: 10.1021/acssynbio.9b00319 Li Cui 1 , Xiaodong Wei 1 , Xinran Wang 1 , Linquan Bai 1 , Shuangjun Lin 1 , Yan Feng 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-01-15 , DOI: 10.1021/acssynbio.9b00319 Li Cui 1 , Xiaodong Wei 1 , Xinran Wang 1 , Linquan Bai 1 , Shuangjun Lin 1 , Yan Feng 1
Affiliation
|
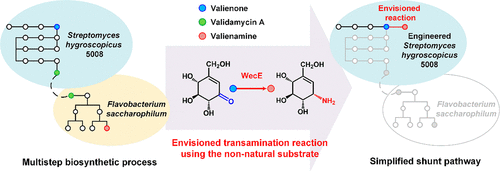
Valienamine is the key functional component of many natural glycosidase inhibitors, including the crop protectant validamycin A and the clinical antidiabetic agent acarbose. Due to its important biomedical activity, it is also the prominent lead compound for the exploration of therapeutic agents, such as the stronger α-glucosidase inhibitor voglibose. Currently, the main route for obtaining valienamine is a multistep biosynthetic process involving the synthesis and degradation of validamycin A. Here, we established an alternative, vastly simplified shunt pathway for the direct synthesis of valienamine based on an envisioned non-natural transamination in the validamycin A producer Streptomyces hygroscopicus 5008. We first identified candidate aminotransferases for the non-natural ketone substrate valienone and conducted molecular evolution in vitro. The WecE enzyme from Escherichia coli was verified to complete the envisioned step with >99.9% enantiomeric excess and was further engineered to produce a 32.6-fold more active mutant, VarB, through protein evolution. Subsequently, two copies of VarB were introduced into the host, and the new shunt pathway produced 0.52 mg/L valienamine after a 96-h fermentation. Our study thus illustrates a dramatically simplified alternative shunt pathway for valienamine production and introduces a promising foundational platform for increasing the production of valienamine and its valuable N-modified derivatives for use in pharmaceutical applications.
中文翻译:

吸水链霉菌5008中缬氨酸胺合成的有效霉素分流途径。
缬氨酸胺是许多天然糖苷酶抑制剂的关键功能成分,包括作物保护剂有效霉素A和临床抗糖尿病药阿卡波糖。由于其重要的生物医学活性,它也是用于探索治疗剂(例如更强的α-葡萄糖苷酶抑制剂伏格列波糖)的重要先导化合物。目前,获得瓦伦胺的主要途径是多步生物合成过程,涉及有效霉素A的合成和降解。在此,我们基于有效霉素中设想的非天然转氨作用,建立了一种替代方法,大大简化了直接合成瓦利胺的分流途径。生产者吸水链霉菌(Streptomyces hygroscopicus)5008。我们首先确定了非天然酮底物缬氨酸的候选氨基转移酶,并在体外进行了分子进化。验证了来自大肠杆菌的WecE酶完成了预想的步骤,对映体过量> 99.9%,并且经过进一步工程改造,可通过蛋白质进化产生32.6倍活性的突变体VarB。随后,将两个拷贝的VarB引入宿主,经过96小时发酵后,新的分流途径产生了0.52 mg / L缬胺。因此,我们的研究说明了缬氨酸胺生产的大大简化的替代分流途径,并为增加缬氨酸胺及其有价值的N-修饰衍生物的生产提供了一个有前途的基础平台,可用于制药应用。通过蛋白质进化。随后,将两个拷贝的VarB引入宿主,经过96小时的发酵后,新的分流途径产生了0.52 mg / L缬胺。因此,我们的研究说明了缬氨酸胺生产的大大简化的替代分流途径,并为增加缬氨酸胺及其有价值的N-修饰衍生物的生产提供了一个有前途的基础平台,可用于制药应用。通过蛋白质进化。随后,将两个拷贝的VarB引入宿主,经过96小时发酵后,新的分流途径产生了0.52 mg / L缬胺。因此,我们的研究说明了缬氨酸胺生产的大大简化的替代分流途径,并介绍了一个有前途的基础平台,用于提高缬氨酸胺及其有价值的N-修饰衍生物的生产,以用于制药应用。
更新日期:2020-01-26
中文翻译:

吸水链霉菌5008中缬氨酸胺合成的有效霉素分流途径。
缬氨酸胺是许多天然糖苷酶抑制剂的关键功能成分,包括作物保护剂有效霉素A和临床抗糖尿病药阿卡波糖。由于其重要的生物医学活性,它也是用于探索治疗剂(例如更强的α-葡萄糖苷酶抑制剂伏格列波糖)的重要先导化合物。目前,获得瓦伦胺的主要途径是多步生物合成过程,涉及有效霉素A的合成和降解。在此,我们基于有效霉素中设想的非天然转氨作用,建立了一种替代方法,大大简化了直接合成瓦利胺的分流途径。生产者吸水链霉菌(Streptomyces hygroscopicus)5008。我们首先确定了非天然酮底物缬氨酸的候选氨基转移酶,并在体外进行了分子进化。验证了来自大肠杆菌的WecE酶完成了预想的步骤,对映体过量> 99.9%,并且经过进一步工程改造,可通过蛋白质进化产生32.6倍活性的突变体VarB。随后,将两个拷贝的VarB引入宿主,经过96小时发酵后,新的分流途径产生了0.52 mg / L缬胺。因此,我们的研究说明了缬氨酸胺生产的大大简化的替代分流途径,并为增加缬氨酸胺及其有价值的N-修饰衍生物的生产提供了一个有前途的基础平台,可用于制药应用。通过蛋白质进化。随后,将两个拷贝的VarB引入宿主,经过96小时的发酵后,新的分流途径产生了0.52 mg / L缬胺。因此,我们的研究说明了缬氨酸胺生产的大大简化的替代分流途径,并为增加缬氨酸胺及其有价值的N-修饰衍生物的生产提供了一个有前途的基础平台,可用于制药应用。通过蛋白质进化。随后,将两个拷贝的VarB引入宿主,经过96小时发酵后,新的分流途径产生了0.52 mg / L缬胺。因此,我们的研究说明了缬氨酸胺生产的大大简化的替代分流途径,并介绍了一个有前途的基础平台,用于提高缬氨酸胺及其有价值的N-修饰衍生物的生产,以用于制药应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号