当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Salicornolides A-C from Gracilaria salicornia attenuate pro-inflammatory 5-lipoxygense: Prospective natural anti-inflammatory leads
Phytochemistry ( IF 3.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.phytochem.2020.112259 Kajal Chakraborty 1 , Tima Antony 2
Phytochemistry ( IF 3.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.phytochem.2020.112259 Kajal Chakraborty 1 , Tima Antony 2
Affiliation
|
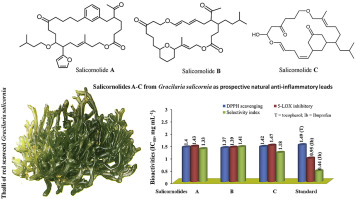
Three macrolides bearing the carbon framework of oxabicyclo[21.3.1]heptacosa-ene-diones (A and B) and oxabicyclo[19.3.1]pentacosa-ene-dione (C) were isolated and characterised from the organic extract of the intertidal red seaweed Gracilaria salicornia (family Gracilariaceae), which were named as salicornolides A-C. These natural macrolides were conformationally pre-organised ring structure providing diverse functionalities, and their potential bioactive properties led to the development of pharmacophores with anti-inflammatory properties. The 21-membered pyran-enclosed salicornolide B displayed greater cyclooxygenase-2 (IC50 COX-2 1.13 mM) inhibitory activity than those exhibited by the 21-membered aryl salicornolide A and 19-membered salicornolide C (IC50 COX-2-1.2 mM). The attenuating potential of the studied compounds against pro-inflammatory enzyme, 5-lipoxygenase (IC50 LOX < 1.5 mM) was significantly greater than that displayed by the non-steroidal anti-inflammatory ibuprofen (IC50 4.5 mM), whereas the selectivity indices exhibited by salicornolides against cyclooxygenase-2 was significantly higher (1.18-1.41, P < 0.05) when compared to that of ibuprofen (SI 0.43) attributing the greater selectivity profile of the former towards inducible pro-inflammatory mediators than the latter. The minimal binding energy of salicornolide B (-9.64 kcal mol-1), a greater number of hydrogen-bonds and lesser inhibitory constant (Ki 85.15 nM) might be responsible for effective binding towards 5-lipoxygenase, and that could attribute its greater anti-inflammation potential than those displayed by other compounds. The putative biosynthetic cascade initiated by malonate-acyl carrier protein unambiguously confirmed the structural attributions of the titled macrocyclic lactones. The undescribed salicornolides A-C from seaweed Gracilaria salicornia attenuating pro-inflammatory 5-lipoxygense might be considered as prospective natural anti-inflammatory leads for pharmaceutical applications.
中文翻译:

来自江蓠的 Salicornolides AC 减弱促炎性 5-脂氧合:潜在的天然抗炎线索
从潮间带红的有机提取物中分离并表征了三种带有氧杂双环[21.3.1]七十二碳烯二酮(A和B)和氧杂双环[19.3.1]五二十碳烯二酮(C)碳骨架的大环内酯海藻Gracilaria salicornia(江蓠科),被命名为salicornolides AC。这些天然大环内酯是构象预组织的环结构,提供多种功能,它们潜在的生物活性特性导致了具有抗炎特性的药效团的发展。与 21 元芳基水杨苷 A 和 19 元水杨苷 C (IC50 COX-2-1.2 mM) 相比,21 元吡喃包裹的水杨苷 B 表现出更大的环加氧酶 2 (IC50 COX-2 1.13 mM) 抑制活性. 所研究的化合物对促炎酶 5-脂氧合酶的减弱潜力(IC50 LOX < 1.5 mM)显着大于非甾体抗炎布洛芬(IC50 4.5 mM)所显示的,而选择性指数由与布洛芬 (SI 0.43) 相比,salicornolides 对环加氧酶-2 的作用显着更高(1.18-1.41,P < 0.05),这归因于前者对诱导性促炎介质的选择性高于后者。水杨酸内酯 B 的最小结合能 (-9.64 kcal mol-1)、更多的氢键和更小的抑制常数 (Ki 85.15 nM) 可能是与 5-脂氧合酶有效结合的原因,这可能归因于其更强的抗- 比其他化合物显示的炎症潜能。由丙二酸-酰基载体蛋白引发的假定生物合成级联明确证实了标题为大环内酯的结构属性。来自海藻 Gracilaria salicornia 的未描述的 salicornolides AC 可减弱促炎性 5-脂氧合酶,可能被认为是用于药物应用的前瞻性天然抗炎先导物。
更新日期:2020-04-01
中文翻译:

来自江蓠的 Salicornolides AC 减弱促炎性 5-脂氧合:潜在的天然抗炎线索
从潮间带红的有机提取物中分离并表征了三种带有氧杂双环[21.3.1]七十二碳烯二酮(A和B)和氧杂双环[19.3.1]五二十碳烯二酮(C)碳骨架的大环内酯海藻Gracilaria salicornia(江蓠科),被命名为salicornolides AC。这些天然大环内酯是构象预组织的环结构,提供多种功能,它们潜在的生物活性特性导致了具有抗炎特性的药效团的发展。与 21 元芳基水杨苷 A 和 19 元水杨苷 C (IC50 COX-2-1.2 mM) 相比,21 元吡喃包裹的水杨苷 B 表现出更大的环加氧酶 2 (IC50 COX-2 1.13 mM) 抑制活性. 所研究的化合物对促炎酶 5-脂氧合酶的减弱潜力(IC50 LOX < 1.5 mM)显着大于非甾体抗炎布洛芬(IC50 4.5 mM)所显示的,而选择性指数由与布洛芬 (SI 0.43) 相比,salicornolides 对环加氧酶-2 的作用显着更高(1.18-1.41,P < 0.05),这归因于前者对诱导性促炎介质的选择性高于后者。水杨酸内酯 B 的最小结合能 (-9.64 kcal mol-1)、更多的氢键和更小的抑制常数 (Ki 85.15 nM) 可能是与 5-脂氧合酶有效结合的原因,这可能归因于其更强的抗- 比其他化合物显示的炎症潜能。由丙二酸-酰基载体蛋白引发的假定生物合成级联明确证实了标题为大环内酯的结构属性。来自海藻 Gracilaria salicornia 的未描述的 salicornolides AC 可减弱促炎性 5-脂氧合酶,可能被认为是用于药物应用的前瞻性天然抗炎先导物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号