当前位置:
X-MOL 学术
›
Eur. J. Pharm. Biopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissolution enhancement of carbamazepine using twin-screw melt granulation.
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.ejpb.2020.01.006 Kristina E Steffens 1 , Karl G Wagner 1
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.ejpb.2020.01.006 Kristina E Steffens 1 , Karl G Wagner 1
Affiliation
|
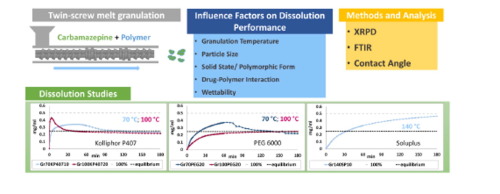
The current study explored the twin-screw melt granulation (TSMG) as a potential technology for the water solubility enhancement of biopharmaceutical classification system (BCS) class II drugs. As a model drug, carbamazepine (CBZ) was formulated with three different polymers as melt granules produced in a co-rotating twin-screw granulator. Polyethylene glycol 6000 (PEG 6000) and Kolliphor® (poloxamer) P407 were used as binding materials at two different granulation temperatures (Tmax: 70 °C; 100 °C). Additionally, Soluplus® (polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer) was chosen as binder of higher melting/ granulation temperature (Tmax: 140 °C). Temperature dependent polymorphic transition of CBZ during melt granulation was observed and identified using XRPD- (X-ray powder diffraction) and FTIR- (Fourier transform infrared spectroscopy) analysis. The effects of polymer type, polymer content (10, 15, 20% (w/w)) and granulation temperature on polymorphic transition, their impact on wettability (contact angle via drop shape-analysis), and the resulting dissolution performance at non-sink conditions in phosphate buffer (pH 6.8), were studied. This study showed that TSMG led to a crystalline system facilitating supersaturation when brought in solution, even when high drug loads (up to 90% (w/w)) were used. In general, for all granules produced, the supersaturation level and its duration varied with the extent of polymorphic transition and binder concentration. The results of this study indicated the importance of temperature control and polymer selection for tailoring desired dissolution profiles.
中文翻译:

使用双螺杆熔融制粒提高卡马西平的溶出度。
当前的研究探索了双螺杆熔体造粒(TSMG)作为增强生物制药分类系统(BCS)II类药物水溶性的潜在技术。作为模型药物,卡马西平(CBZ)与三种不同的聚合物一起配制为在同向旋转双螺杆制粒机中生产的熔体颗粒。聚乙二醇6000(PEG 6000)和(泊洛沙姆)P407在两种不同的制粒温度(Tmax:70°C; 100°C)下用作粘合材料。此外,Soluplus®(聚乙烯基己内酰胺-聚乙酸乙烯酯-聚乙二醇接枝共聚物)被选作具有更高熔融/制粒温度(最高温度:140°C)的粘合剂。使用XRPD-(X射线粉末衍射)和FTIR-(傅里叶变换红外光谱)分析观察并鉴定了熔融制粒过程中CBZ的温度依赖性多晶型转变。聚合物类型,聚合物含量(10%,15%,20%(w / w))和制粒温度对多晶型转变的影响,它们对润湿性的影响(通过液滴形状分析的接触角),以及在非饱和状态下的溶解性能研究了磷酸盐缓冲液(pH 6.8)中的下沉条件。这项研究表明,即使使用高载药量(高达90%(w / w)),TSMG引入溶液时,也会导致促进过饱和的晶体系统。通常,对于所有生产的颗粒,过饱和水平及其持续时间随多晶型转变程度和粘合剂浓度而变化。
更新日期:2020-01-16
中文翻译:

使用双螺杆熔融制粒提高卡马西平的溶出度。
当前的研究探索了双螺杆熔体造粒(TSMG)作为增强生物制药分类系统(BCS)II类药物水溶性的潜在技术。作为模型药物,卡马西平(CBZ)与三种不同的聚合物一起配制为在同向旋转双螺杆制粒机中生产的熔体颗粒。聚乙二醇6000(PEG 6000)和(泊洛沙姆)P407在两种不同的制粒温度(Tmax:70°C; 100°C)下用作粘合材料。此外,Soluplus®(聚乙烯基己内酰胺-聚乙酸乙烯酯-聚乙二醇接枝共聚物)被选作具有更高熔融/制粒温度(最高温度:140°C)的粘合剂。使用XRPD-(X射线粉末衍射)和FTIR-(傅里叶变换红外光谱)分析观察并鉴定了熔融制粒过程中CBZ的温度依赖性多晶型转变。聚合物类型,聚合物含量(10%,15%,20%(w / w))和制粒温度对多晶型转变的影响,它们对润湿性的影响(通过液滴形状分析的接触角),以及在非饱和状态下的溶解性能研究了磷酸盐缓冲液(pH 6.8)中的下沉条件。这项研究表明,即使使用高载药量(高达90%(w / w)),TSMG引入溶液时,也会导致促进过饱和的晶体系统。通常,对于所有生产的颗粒,过饱和水平及其持续时间随多晶型转变程度和粘合剂浓度而变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号