Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
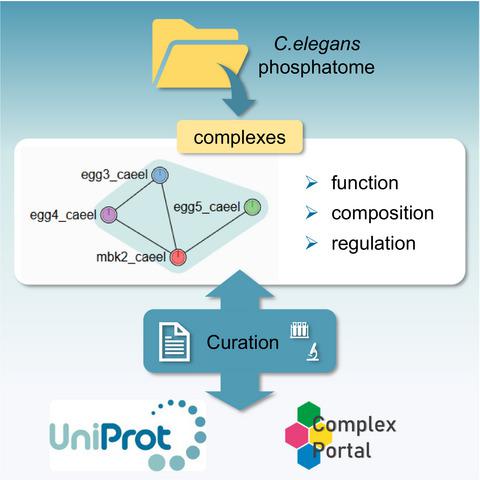
Caenorhabditis elegans phosphatase complexes in UniProtKB and Complex Portal.
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1111/febs.15213 Hema Bye-A-Jee 1 , Rossana Zaru 1 , Michele Magrane 1 , Sandra Orchard 1 , 1, 2, 3, 4
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-16 , DOI: 10.1111/febs.15213 Hema Bye-A-Jee 1 , Rossana Zaru 1 , Michele Magrane 1 , Sandra Orchard 1 , 1, 2, 3, 4
Affiliation
|
Phosphatases play an essential role in the regulation of protein phosphorylation. Less abundant than kinases, many phosphatases are components of one or more macromolecular complexes with different substrate specificities and specific functionalities. The expert scientific curation of phosphatase complexes for the UniProt and Complex Portal databases supports the whole scientific community by collating and organising small‐ and large‐scale experimental data from the scientific literature into context‐specific central resources, where the data can be freely accessed and used to further academic and translational research. In this review, we discuss how the diverse biological functions of phosphatase complexes are presented in UniProt and the Complex Portal, and how understanding the biological significance of phosphatase complexes in Caenorhabditis elegans offers insight into the mechanisms of substrate diversity in a variety of cellular and molecular processes.
中文翻译:

秀丽隐杆线虫磷酸酶复合物位于UniProtKB和Complex Portal中。
磷酸酶在蛋白质磷酸化的调节中起着至关重要的作用。磷酸酶的含量比激酶少,它是一种或多种具有不同底物特异性和特异性功能的大分子复合物的成分。UniProt和Complex Portal数据库的磷酸酶复合物的专家科学管理通过将科学文献中的小规模和大规模实验数据整理和组织到特定于上下文的中央资源中,从而为整个科学界提供支持,可以自由访问和用于进一步的学术和翻译研究。在这篇综述中,我们讨论了如何在UniProt和Complex Portal中介绍磷酸酶复合物的多种生物学功能,以及如何理解磷酸酶复合物在体内的生物学意义。秀丽隐杆线虫可以洞察各种细胞和分子过程中底物多样性的机制。
更新日期:2020-01-16
中文翻译:

秀丽隐杆线虫磷酸酶复合物位于UniProtKB和Complex Portal中。
磷酸酶在蛋白质磷酸化的调节中起着至关重要的作用。磷酸酶的含量比激酶少,它是一种或多种具有不同底物特异性和特异性功能的大分子复合物的成分。UniProt和Complex Portal数据库的磷酸酶复合物的专家科学管理通过将科学文献中的小规模和大规模实验数据整理和组织到特定于上下文的中央资源中,从而为整个科学界提供支持,可以自由访问和用于进一步的学术和翻译研究。在这篇综述中,我们讨论了如何在UniProt和Complex Portal中介绍磷酸酶复合物的多种生物学功能,以及如何理解磷酸酶复合物在体内的生物学意义。秀丽隐杆线虫可以洞察各种细胞和分子过程中底物多样性的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号