当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An example of how to establish the thermodynamic stability relationship between two polymorphs of a compound highly prone to solvate formation.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.ejps.2020.105215 Norbert Nagel 1 , Bruno Baumgartner 1 , Harald Berchtold 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.ejps.2020.105215 Norbert Nagel 1 , Bruno Baumgartner 1 , Harald Berchtold 1
Affiliation
|
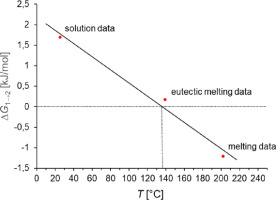
Upon transition from research to development, a new chemical entity, which acts upon the Kv1.5-potassium channel and blocks potassium flow in the atrium of the human heart, has been subjected to a crystallization screen. The sodium salt of an anthranilic acid amide with a heteroarylsulfonyl side chain forms solvates from all tested organic solvents. Solvent-free crystalline phases can only be obtained by drying certain solvates under suitable conditions. Two well crystalline solvent-free phases can be obtained this way. Three different methods were applied to determine their thermodynamic stability relationship from melting, solution and eutectic melting data. The different approaches are discussed and compared with respect to their accuracy and limitations.
中文翻译:

如何在高度易于形成溶剂化物的化合物的两个多晶型物之间建立热力学稳定性关系的示例。
从研究到开发的过渡阶段,作用于Kv1.5-钾通道并阻断钾在人心房中流动的新化学实体已经过结晶筛选。具有杂芳基磺酰基侧链的邻氨基苯甲酰胺的钠盐从所有测试的有机溶剂中形成溶剂化物。无溶剂的结晶相只能通过在适当的条件下干燥某些溶剂化物来获得。可以通过这种方式获得两个良好结晶的无溶剂相。应用三种不同的方法从熔融,固溶和低共熔数据确定其热力学稳定性关系。讨论并比较了不同方法的准确性和局限性。
更新日期:2020-01-16
中文翻译:

如何在高度易于形成溶剂化物的化合物的两个多晶型物之间建立热力学稳定性关系的示例。
从研究到开发的过渡阶段,作用于Kv1.5-钾通道并阻断钾在人心房中流动的新化学实体已经过结晶筛选。具有杂芳基磺酰基侧链的邻氨基苯甲酰胺的钠盐从所有测试的有机溶剂中形成溶剂化物。无溶剂的结晶相只能通过在适当的条件下干燥某些溶剂化物来获得。可以通过这种方式获得两个良好结晶的无溶剂相。应用三种不同的方法从熔融,固溶和低共熔数据确定其热力学稳定性关系。讨论并比较了不同方法的准确性和局限性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号