当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypoxia-sensitive supramolecular nanogels for the cytosolic delivery of ribonuclease A as a breast cancer therapeutic.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.jconrel.2020.01.021 Xinghui Si 1 , Sheng Ma 1 , Yudi Xu 2 , Dawei Zhang 3 , Na Shen 3 , Haiyang Yu 3 , Yu Zhang 3 , Wantong Song 3 , Zhaohui Tang 4 , Xuesi Chen 3
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.jconrel.2020.01.021 Xinghui Si 1 , Sheng Ma 1 , Yudi Xu 2 , Dawei Zhang 3 , Na Shen 3 , Haiyang Yu 3 , Yu Zhang 3 , Wantong Song 3 , Zhaohui Tang 4 , Xuesi Chen 3
Affiliation
|
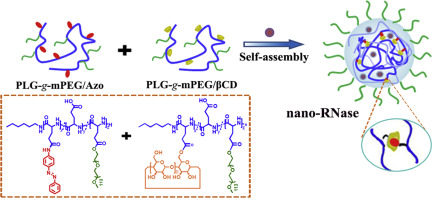
As the most common malignancy in women, breast cancer causes >40,000 deaths annually. Ribonuclease A (RNase), a new anti-cancer agent, has attracted intense interest due to its high efficacy and specificity. However, RNase suffers from instability, a short half-life in the circulation and poor membrane penetration. To overcome these challenges, we designed a supramolecular nanogel for the cytosolic delivery of RNase. The nanogels were fabricated using host-guest interactions between azobenzene (Azo) and β-cyclodextrin (βCD) conjugated to poly (L-glutamic acid)-graft-poly (ethylene glycol) methyl ether (PLG-g-mPEG). RNase could be loaded inside the nanogels in mild aqueous conditions. Following optimization, the RNase-loading content and efficiency of the nanogel were 23.5 wt% and 50.4%, respectively. In the presence of nitroreductase (NTR), the cross-linking point between Azo and βCD was destroyed due to the conformation transition of Azo, ensuring the hypoxia-sensitive release of cargo from the nanogels in tumors in which NTR is overexpressed. In vitro release profiles revealed that 75.0% of the RNase was released under hypoxic conditions in 72 h, whilst only 19.7% was released under normoxic conditions. Cytotoxicity assays showed that the RNase-loaded nanogels (nano-RNase) were more efficient in inhibiting the proliferation of 4T1 cells than free RNase. In vivo studies showed 68.7% tumor suppression rates (TSR %) in the nano-RNase treated group, whilst free RNase treatment led to a lack of tumor inhibition. To further enhance the hypoxia status of tumors, we combined nano-RNase with a nanoformulation of vascular disrupting agents PLG-g-mPEG/combretastatinA4 (nano-CA4) and obtained a TSR of 91.7%. The hypoxia-sensitive supramolecular nanogels provided a versatile platform for the delivery of RNase, highlighting its applicability for cancer therapy.
中文翻译:

低氧敏感性超分子纳米凝胶,用于核糖核酸酶A的胞质递送,作为乳腺癌治疗剂。
乳腺癌是女性最常见的恶性肿瘤,每年导致40,000多例死亡。核糖核酸酶A(RNase)是一种新型的抗癌药,由于其高功效和高特异性而引起了广泛的关注。但是,RNase具有不稳定,循环半衰期短和膜渗透性差的缺点。为了克服这些挑战,我们设计了一种用于RNase胞质递送的超分子纳米凝胶。使用偶氮苯(Azo)和与聚(L-谷氨酸)-接枝-聚(乙二醇)甲基醚(PLG-g-mPEG)共轭的β-环糊精(βCD)之间的主客体相互作用制备纳米凝胶。RNase可以在温和的水性条件下加载到纳米凝胶中。经过优化后,纳米凝胶的RNase负载量和效率分别为23.5 wt%和50.4%。在存在硝基还原酶(NTR)的情况下,由于偶氮的构象转变,偶氮与βCD之间的交联点被破坏,从而确保了NTR过表达的肿瘤中纳米凝胶对货物的低氧敏感性释放。体外释放曲线表明,在缺氧条件下72小时内释放了75.0%的RNase,而在常氧条件下仅释放了19.7%。细胞毒性试验表明,负载RNase的纳米凝胶(纳米RNase)比游离RNase更有效地抑制4T1细胞的增殖。体内研究显示,纳米RNase治疗组的肿瘤抑制率为68.7%(TSR%),而免费RNase治疗导致缺乏肿瘤抑制作用。为了进一步增强肿瘤的缺氧状态,我们将纳米RNA酶与血管分裂剂PLG-g-mPEG / combretastatinA4(nano-CA4)的纳米制剂结合使用,获得的TSR为91.7%。对缺氧敏感的超分子纳米凝胶为RNase的递送提供了一个通用平台,突出了其在癌症治疗中的适用性。
更新日期:2020-01-16
中文翻译:

低氧敏感性超分子纳米凝胶,用于核糖核酸酶A的胞质递送,作为乳腺癌治疗剂。
乳腺癌是女性最常见的恶性肿瘤,每年导致40,000多例死亡。核糖核酸酶A(RNase)是一种新型的抗癌药,由于其高功效和高特异性而引起了广泛的关注。但是,RNase具有不稳定,循环半衰期短和膜渗透性差的缺点。为了克服这些挑战,我们设计了一种用于RNase胞质递送的超分子纳米凝胶。使用偶氮苯(Azo)和与聚(L-谷氨酸)-接枝-聚(乙二醇)甲基醚(PLG-g-mPEG)共轭的β-环糊精(βCD)之间的主客体相互作用制备纳米凝胶。RNase可以在温和的水性条件下加载到纳米凝胶中。经过优化后,纳米凝胶的RNase负载量和效率分别为23.5 wt%和50.4%。在存在硝基还原酶(NTR)的情况下,由于偶氮的构象转变,偶氮与βCD之间的交联点被破坏,从而确保了NTR过表达的肿瘤中纳米凝胶对货物的低氧敏感性释放。体外释放曲线表明,在缺氧条件下72小时内释放了75.0%的RNase,而在常氧条件下仅释放了19.7%。细胞毒性试验表明,负载RNase的纳米凝胶(纳米RNase)比游离RNase更有效地抑制4T1细胞的增殖。体内研究显示,纳米RNase治疗组的肿瘤抑制率为68.7%(TSR%),而免费RNase治疗导致缺乏肿瘤抑制作用。为了进一步增强肿瘤的缺氧状态,我们将纳米RNA酶与血管分裂剂PLG-g-mPEG / combretastatinA4(nano-CA4)的纳米制剂结合使用,获得的TSR为91.7%。对缺氧敏感的超分子纳米凝胶为RNase的递送提供了一个通用平台,突出了其在癌症治疗中的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号