Water Research ( IF 11.4 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.watres.2020.115508 Shiqing Zhou , Yangtao Wu , Shumin Zhu , Julong Sun , Lingjun Bu , Dionysios D. Dionysiou
|
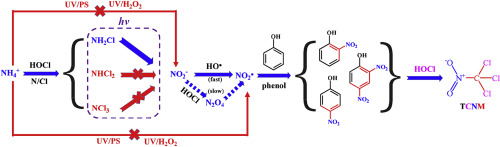
In this study, the potential formation of trichloronitromethane (TCNM) from model organic compounds in ammonia-containing water treated by UV/chlorine process was evaluated. Monochloramine generated from the reaction of chlorine and ammonia can be photolyzed to produce NO2− and reactive nitrogen species (RNS), which play important roles in the formation of TCNM during the subsequent chlorination. The results showed that increase of nitrogen to chlorine molar ratio (from 0 to 1.0) and pH (from 6.5 to 8.0) enhanced the formation of TCNM, mainly due to the increased yield of NO2− and RNS from the photolyzed monochloramine. The formation of TCNM was interestingly found to be linearly correlated with Hammett constants of the model precursors, which is theoretically related to the rate constants of RNS with model compounds. Enhanced formation of TCNM was also observed during the treatment of natural organic matter by UV/chlorine process in ammonia-containing water. The toxicity assessment showed that TCNM significantly increased the genotoxicity of formed DBPs. Furthermore, the electrophilic substitution reaction of •NO2 was proved to more likely occur on the ortho and para position of phenol according to the calculation of Gaussian program, and a possible reaction pathway of phenol and •NO2 was proposed based on the calculated results.
中文翻译:

氮从氨到三氯硝基甲烷的转化:UV /氯过程中的潜在风险
在这项研究中,评估了由模型有机化合物在经过UV /氯处理的含氨水中可能形成的三氯硝基甲烷(TCNM)。选自氯和氨的反应所产生的一氯胺可光分解而产生NO 2 -和活性氮类(RNS),这随后的氯化处理时TCNM的形成中发挥重要作用。结果表明,氮与氯的摩尔比(从0到1.0)和pH(从6.5到8.0)的增加会增强TCNM的形成,这主要是由于NO 2-的产率增加所致。和RNS来自光解的一氯胺。有趣的是,发现TCNM的形成与模型前体的Hammett常数线性相关,这在理论上与RNS与模型化合物的速率常数相关。在含氨水中通过紫外线/氯气处理天然有机物的过程中,也观察到TCNM的形成增加。毒性评估表明,TCNM显着增加了所形成DBP的遗传毒性。此外,根据高斯程序的计算,证明了• NO 2的亲电取代反应更可能发生在苯酚的邻位和对位,以及苯酚和•根据计算结果提出了NO 2。









































 京公网安备 11010802027423号
京公网安备 11010802027423号