当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-catalyzed asymmetric hydrogenation of exocyclic α,β-unsaturated carbonyl compounds.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-15 , DOI: 10.1039/c9ob02536g Jiaxin Yang 1 , Xiuxiu Li 1 , Cai You 1 , Shuailong Li 1 , Yu-Qing Guan 1 , Hui Lv 1 , Xumu Zhang 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-15 , DOI: 10.1039/c9ob02536g Jiaxin Yang 1 , Xiuxiu Li 1 , Cai You 1 , Shuailong Li 1 , Yu-Qing Guan 1 , Hui Lv 1 , Xumu Zhang 2
Affiliation
|
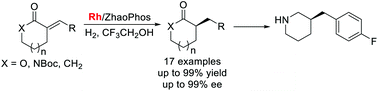
A highly enantioselective hydrogenation of exocyclic α,β-unsaturated carbonyl compounds catalyzed by Rh/bisphosphine-thiourea (ZhaoPhos) has been developed, giving the corresponding α-chiral cyclic lactones, lactams and ketones with high yields and excellent enantioselectivities (up to 99% yield and 99% ee). Remarkably, the hydrogen bond between the substrate and the catalyst plays a critical role in this transformation. The synthetic utility of this protocol has been demonstrated by efficient synthesis of chiral 3-(4-fluorobenzyl)piperidine, a key chiral fragment of bioactive molecules.
中文翻译:

铑催化的环外α,β-不饱和羰基化合物的不对称氢化。
已开发出由Rh /双膦-硫脲(ZhaoPhos)催化的对环外α,β-不饱和羰基化合物的高度对映选择性氢化,可提供相应的α-手性环状内酯,内酰胺和酮,产率高且对映选择性极好(高达99%收率和99%ee)。显着地,底物和催化剂之间的氢键在该转变中起关键作用。该协议的合成用途已通过有效合成手性3-(4-氟苄基)哌啶(一种生物活性分子的关键手性片段)得到了证明。
更新日期:2020-02-13
中文翻译:

铑催化的环外α,β-不饱和羰基化合物的不对称氢化。
已开发出由Rh /双膦-硫脲(ZhaoPhos)催化的对环外α,β-不饱和羰基化合物的高度对映选择性氢化,可提供相应的α-手性环状内酯,内酰胺和酮,产率高且对映选择性极好(高达99%收率和99%ee)。显着地,底物和催化剂之间的氢键在该转变中起关键作用。该协议的合成用途已通过有效合成手性3-(4-氟苄基)哌啶(一种生物活性分子的关键手性片段)得到了证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号