Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, eco-friendly synthesis, molecular modeling and anticancer evaluation of thiazol-5(4H)-ones as potential tubulin polymerization inhibitors targeting the colchicine binding site
RSC Advances ( IF 3.9 ) Pub Date : 2020-1-15 , DOI: 10.1039/c9ra10094f Abeer M El-Naggar 1 , Ibrahim H Eissa 2 , Amany Belal 3 , Amira A El-Sayed 1
RSC Advances ( IF 3.9 ) Pub Date : 2020-1-15 , DOI: 10.1039/c9ra10094f Abeer M El-Naggar 1 , Ibrahim H Eissa 2 , Amany Belal 3 , Amira A El-Sayed 1
Affiliation
|
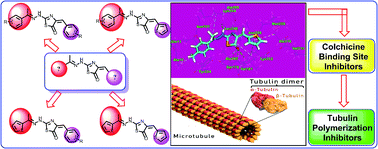
In recent years, suppressing tubulin polymerization has been developed as a therapeutic approach for cancer treatment. Thus, new derivatives based on thiazol-5(4H)-ones have been designed and synthesized in an eco-friendly manner. The synthesized derivatives have the same essential pharmacophoric features of colchicine binding site inhibitors. The anti-proliferative activity of the new derivatives was evaluated on three human cancer cell lines (HCT-116, HepG-2, and MCF-7) using MTT assay procedure and colchicine was used as a positive control. Compounds 4f, 5a, 8f, 8g, and 8k showed superior antiproliferative activities against the three tested cell lines with IC50 values ranging from 2.89 to 9.29 μM. Further investigation for the most active cytotoxic agents as tubulin polymerization inhibitors was also performed in order to explore the mechanism of their anti-proliferative activity. Tubulin polymerization assay results were found to be comperable with the cytotoxicity results. Compounds 4f and 5a were the most potent tubulin polymerization inhibitors with an IC50 value of 9.33 and 9.52 nM, respectively. Further studies revealed the ability of 5a to induce apoptosis and arrest cell cycle growth at the G2/M phase. Molecular docking studies were also conducted to investigate possible binding interactions between the target compounds and the tubulin heterodimer active site. From these studies, it was concluded that inhibition of tubulin polymerization yields the reported cytotoxic activity.
中文翻译:

thiazol-5(4H)-ones 作为潜在的针对秋水仙碱结合位点的微管蛋白聚合抑制剂的设计、环保合成、分子建模和抗癌评价
近年来,抑制微管蛋白聚合已被开发为癌症治疗的治疗方法。因此,基于 thiazol-5(4 H )-ones 的新衍生物已被设计并以环保的方式合成。合成的衍生物具有与秋水仙碱结合位点抑制剂相同的基本药效特征。使用 MTT 测定程序评估了新衍生物对三种人类癌细胞系(HCT-116、HepG-2 和 MCF-7)的抗增殖活性,并使用秋水仙碱作为阳性对照。化合物4f、5a、8f、8g和8k对三种测试的细胞系显示出优异的抗增殖活性,IC 50值范围为 2.89 至 9.29 μM。还对最活跃的细胞毒剂作为微管蛋白聚合抑制剂进行了进一步研究,以探索其抗增殖活性的机制。发现微管蛋白聚合测定结果与细胞毒性结果相当。化合物4f和5a是最有效的微管蛋白聚合抑制剂,IC 50值分别为 9.33 和 9.52 nM。进一步的研究揭示了5a的能力在 G2/M 期诱导细胞凋亡并阻止细胞周期的生长。还进行了分子对接研究以研究目标化合物与微管蛋白异二聚体活性位点之间可能的结合相互作用。从这些研究中可以得出结论,抑制微管蛋白聚合产生了报告的细胞毒活性。
更新日期:2020-01-15
中文翻译:

thiazol-5(4H)-ones 作为潜在的针对秋水仙碱结合位点的微管蛋白聚合抑制剂的设计、环保合成、分子建模和抗癌评价
近年来,抑制微管蛋白聚合已被开发为癌症治疗的治疗方法。因此,基于 thiazol-5(4 H )-ones 的新衍生物已被设计并以环保的方式合成。合成的衍生物具有与秋水仙碱结合位点抑制剂相同的基本药效特征。使用 MTT 测定程序评估了新衍生物对三种人类癌细胞系(HCT-116、HepG-2 和 MCF-7)的抗增殖活性,并使用秋水仙碱作为阳性对照。化合物4f、5a、8f、8g和8k对三种测试的细胞系显示出优异的抗增殖活性,IC 50值范围为 2.89 至 9.29 μM。还对最活跃的细胞毒剂作为微管蛋白聚合抑制剂进行了进一步研究,以探索其抗增殖活性的机制。发现微管蛋白聚合测定结果与细胞毒性结果相当。化合物4f和5a是最有效的微管蛋白聚合抑制剂,IC 50值分别为 9.33 和 9.52 nM。进一步的研究揭示了5a的能力在 G2/M 期诱导细胞凋亡并阻止细胞周期的生长。还进行了分子对接研究以研究目标化合物与微管蛋白异二聚体活性位点之间可能的结合相互作用。从这些研究中可以得出结论,抑制微管蛋白聚合产生了报告的细胞毒活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号