当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
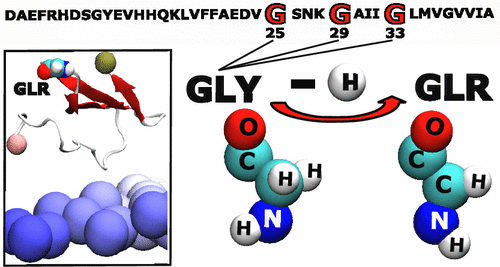
Role of Oxidized Gly25, Gly29, and Gly33 Residues on the Interactions of Aβ1-42 with Lipid Membranes.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-30 , DOI: 10.1021/acschemneuro.9b00558 Hebah Fatafta 1 , Chetan Poojari 2, 3 , Abdallah Sayyed-Ahmad 4 , Birgit Strodel 1, 5 , Michael C Owen 6
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-01-30 , DOI: 10.1021/acschemneuro.9b00558 Hebah Fatafta 1 , Chetan Poojari 2, 3 , Abdallah Sayyed-Ahmad 4 , Birgit Strodel 1, 5 , Michael C Owen 6
Affiliation
|
Oxidative stress is known to play an important role in the pathogenesis of Alzheimer's disease. Moreover, it is becoming increasingly evident that the plasma membrane of neurons plays a role in modulating the aggregation and toxicity of Alzheimer's amyloid-β peptide (Aβ). In this study, the combined and interdependent effects of oxidation and membrane interactions on the 42 residues long Aβ isoform are investigated using molecular simulations. Hamiltonian replica exchange molecular dynamics simulations are utilized to elucidate the impact of selected oxidized glycine residues of Aβ42 on the interactions of the peptide with a model membrane comprised of 70% POPC, 25% cholesterol, and 5% of the ganglioside GM1. The main findings are that, independent of the oxidation state, Aβ prefers binding to GM1 over POPC, which is further enhanced by the oxidation of Gly29 and Gly33 and reduced the formation of β-sheet. Our results suggest that the differences observed in Aβ42 conformations and its interaction with a lipid bilayer upon oxidation originate from the position of the oxidized Gly residue with respect to the hydrophobic sequence of Aβ42 involving the Gly29-XXX-Gly33-XXX-Gly37 motif and from specific interactions between the peptide and the terminal sugar groups of GM1.
中文翻译:

氧化的Gly25,Gly29和Gly33残基在Aβ1-42与脂质膜相互作用中的作用。
已知氧化应激在阿尔茨海默氏病的发病机理中起重要作用。而且,越来越明显的是,神经元的质膜在调节阿尔茨海默氏淀粉样β肽(Aβ)的聚集和毒性中起作用。在这项研究中,使用分子模拟研究了氧化和膜相互作用对42个残基长的Aβ同工型的联合和相互依赖的影响。汉密尔顿复制品交换分子动力学模拟用于阐明Aβ42氧化甘氨酸残基对肽与包含70%POPC,25%胆固醇和5%神经节苷脂GM1的模型膜相互作用的影响。主要发现是,与氧化态无关,Aβ较POPC更喜欢与GM1结合,Gly29和Gly33的氧化作用进一步增强了这种作用,并减少了β-折叠的形成。我们的结果表明,观察到的Aβ42构象及其与脂质双层相互作用的差异源自氧化的Gly残基相对于涉及Gly29-XXX-Gly33-XXX-Gly37基序的Aβ42疏水序列的位置。肽和GM1末端糖基之间的特定相互作用。
更新日期:2020-01-31
中文翻译:

氧化的Gly25,Gly29和Gly33残基在Aβ1-42与脂质膜相互作用中的作用。
已知氧化应激在阿尔茨海默氏病的发病机理中起重要作用。而且,越来越明显的是,神经元的质膜在调节阿尔茨海默氏淀粉样β肽(Aβ)的聚集和毒性中起作用。在这项研究中,使用分子模拟研究了氧化和膜相互作用对42个残基长的Aβ同工型的联合和相互依赖的影响。汉密尔顿复制品交换分子动力学模拟用于阐明Aβ42氧化甘氨酸残基对肽与包含70%POPC,25%胆固醇和5%神经节苷脂GM1的模型膜相互作用的影响。主要发现是,与氧化态无关,Aβ较POPC更喜欢与GM1结合,Gly29和Gly33的氧化作用进一步增强了这种作用,并减少了β-折叠的形成。我们的结果表明,观察到的Aβ42构象及其与脂质双层相互作用的差异源自氧化的Gly残基相对于涉及Gly29-XXX-Gly33-XXX-Gly37基序的Aβ42疏水序列的位置。肽和GM1末端糖基之间的特定相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号