当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polarization of Macrophages, Cellular Adhesion, and Spreading on Bacterially Contaminated Gold Nanoparticle-Coatings in Vitro
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-01-29 , DOI: 10.1021/acsbiomaterials.9b01518 Yafei Luan 1, 2 , Henny C. van der Mei 2 , Melissa Dijk 3 , Gésinda I. Geertsema-Doornbusch 2 , Jelly Atema-Smit 2 , Yijin Ren 3 , Hong Chen 1 , Henk J. Busscher 2
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-01-29 , DOI: 10.1021/acsbiomaterials.9b01518 Yafei Luan 1, 2 , Henny C. van der Mei 2 , Melissa Dijk 3 , Gésinda I. Geertsema-Doornbusch 2 , Jelly Atema-Smit 2 , Yijin Ren 3 , Hong Chen 1 , Henk J. Busscher 2
Affiliation
|
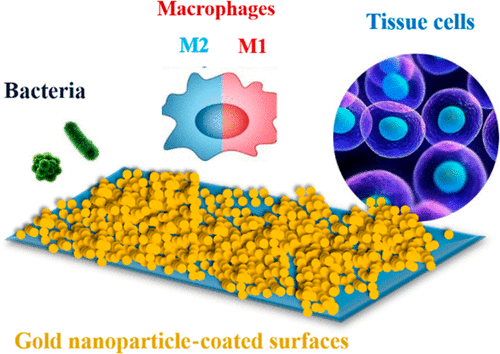
Biomaterial-associated infections often arise from contaminating bacteria adhering to an implant surface that are introduced during surgical implantation and not effectively eradicated by antibiotic treatment. Whether or not infection develops from contaminating bacteria depends on an interplay between bacteria contaminating the biomaterial surface and tissue cells trying to integrate the surface with the aid of immune cells. The biomaterial surface plays a crucial role in defining the outcome of this race for the surface. Tissue integration is considered the best protection of a biomaterial implant against infectious bacteria. This paper aims to determine whether and how macrophages aid osteoblasts and human mesenchymal stem cells to adhere and spread over gold nanoparticle (GNP)-coatings with different hydrophilicity and roughness in the absence or presence of contaminating, adhering bacteria. All GNP-coatings had identical chemical surface composition, and water contact angles decreased with increasing roughness. Upon increasing the roughness of the GNP-coatings, the presence of contaminating Staphylococcus epidermidis in biculture with cells gradually decreased surface coverage by adhering and spreading cells, as in the absence of staphylococci. More virulent Staphylococcus aureus fully impeded cellular adhesion and spreading on smooth gold- or GNP-coatings, while Escherichia coli allowed minor cellular interaction. Murine macrophages in monoculture tended toward their pro-inflammatory “fighting” M1-phenotype on all coatings to combat the biomaterial, but in bicultures with contaminating, adhering bacteria, macrophages demonstrated Ym1 expression, indicative of polarization toward their anti-inflammatory “fix-and-repair” M2-phenotype. Damage repair of cells by macrophages improved cellular interactions on intermediately hydrophilic/rough (water contact angle 30 deg/surface roughness 118 nm) GNP-coatings in the presence of contaminating, adhering Gram-positive staphylococci but provided little aid in the presence of Gram-negative E. coli. Thus, the merits on GNP-coatings to influence the race for the surface and prevent biomaterial-associated infection critically depend on their hydrophilicity/roughness and the bacterial strain involved in contaminating the biomaterial surface.
中文翻译:

巨噬细胞的极化,细胞粘附和体外细菌污染的金纳米粒子涂层上的传播。
与生物材料相关的感染通常是由于污染附着在植入物表面的细菌而引起的,这些细菌是在手术植入过程中引入的,并不能通过抗生素治疗有效消除。感染是否由污染细菌引起,取决于污染生物材料表面的细菌与试图借助免疫细胞整合表面的组织细胞之间的相互作用。生物材料表面在决定表面种族的结果中起着至关重要的作用。组织整合被认为是生物材料植入物抵抗传染细菌的最佳保护。本文旨在确定巨噬细胞是否以及如何在不存在或存在污染性粘附细菌的情况下,帮助成骨细胞和人间充质干细胞粘附并扩散在具有不同亲水性和粗糙度的金纳米颗粒(GNP)涂层上。所有GNP涂层的化学表面成分均相同,并且水的接触角随粗糙度的增加而减小。随着GNP涂层粗糙度的增加,污染物的存在在不存在葡萄球菌的情况下,与细胞一起培养的表皮葡萄球菌通过粘附和扩散细胞逐渐降低了表面覆盖率。更具毒性的金黄色葡萄球菌完全阻碍细胞粘附并在光滑的金或GNP涂层上扩散,而大肠杆菌允许较小的细胞相互作用。单一培养中的小鼠巨噬细胞倾向于在所有涂层上都具有促炎性的“战斗” M1-表型,以对抗生物材料,但是在带有污染,粘附细菌的双重培养中,巨噬细胞表现出Ym1表达,表明它们朝着消炎性“固定和极化”方向分化。 -修复” M2-表型。巨噬细胞对细胞的损伤修复改善了在存在污染,粘附革兰氏阳性葡萄球菌的情况下中等亲水/粗糙(水接触角30度/表面粗糙度118 nm)GNP涂层上的细胞相互作用,但在存在革兰氏阳性菌时几乎没有帮助大肠杆菌阴性。因此,在GNP涂层上影响表面种族并防止生物材料相关感染的优点主要取决于它们的亲水性/粗糙度和污染生物材料表面的细菌菌株。
更新日期:2020-01-29
中文翻译:

巨噬细胞的极化,细胞粘附和体外细菌污染的金纳米粒子涂层上的传播。
与生物材料相关的感染通常是由于污染附着在植入物表面的细菌而引起的,这些细菌是在手术植入过程中引入的,并不能通过抗生素治疗有效消除。感染是否由污染细菌引起,取决于污染生物材料表面的细菌与试图借助免疫细胞整合表面的组织细胞之间的相互作用。生物材料表面在决定表面种族的结果中起着至关重要的作用。组织整合被认为是生物材料植入物抵抗传染细菌的最佳保护。本文旨在确定巨噬细胞是否以及如何在不存在或存在污染性粘附细菌的情况下,帮助成骨细胞和人间充质干细胞粘附并扩散在具有不同亲水性和粗糙度的金纳米颗粒(GNP)涂层上。所有GNP涂层的化学表面成分均相同,并且水的接触角随粗糙度的增加而减小。随着GNP涂层粗糙度的增加,污染物的存在在不存在葡萄球菌的情况下,与细胞一起培养的表皮葡萄球菌通过粘附和扩散细胞逐渐降低了表面覆盖率。更具毒性的金黄色葡萄球菌完全阻碍细胞粘附并在光滑的金或GNP涂层上扩散,而大肠杆菌允许较小的细胞相互作用。单一培养中的小鼠巨噬细胞倾向于在所有涂层上都具有促炎性的“战斗” M1-表型,以对抗生物材料,但是在带有污染,粘附细菌的双重培养中,巨噬细胞表现出Ym1表达,表明它们朝着消炎性“固定和极化”方向分化。 -修复” M2-表型。巨噬细胞对细胞的损伤修复改善了在存在污染,粘附革兰氏阳性葡萄球菌的情况下中等亲水/粗糙(水接触角30度/表面粗糙度118 nm)GNP涂层上的细胞相互作用,但在存在革兰氏阳性菌时几乎没有帮助大肠杆菌阴性。因此,在GNP涂层上影响表面种族并防止生物材料相关感染的优点主要取决于它们的亲水性/粗糙度和污染生物材料表面的细菌菌株。











































 京公网安备 11010802027423号
京公网安备 11010802027423号