当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and biological screening of some novel 6‐substituted 2‐alkylpyridazin‐3(2 H )‐ones as anti‐inflammatory and analgesic agents
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-01-15 , DOI: 10.1002/ardp.201900295 Yasser M Loksha 1 , Mohammad M Abd-Alhaseeb 2
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-01-15 , DOI: 10.1002/ardp.201900295 Yasser M Loksha 1 , Mohammad M Abd-Alhaseeb 2
Affiliation
|
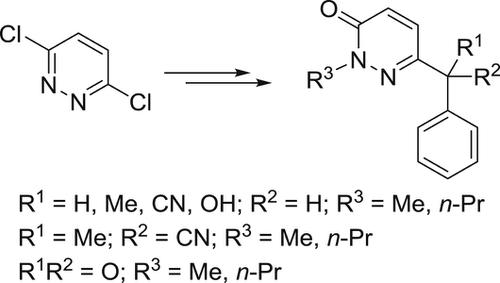
Some novel derivatives of 2‐alkyl 6‐substituted pyridazin‐3(2H)‐ones were synthesized by condensation of 3,6‐dichloropyridazine with the sodium salt of benzyl cyanide, followed by hydrolysis and coupling with alkyl halides. The synthesized compounds were screened as cyclooxygenase (COX)‐1/COX‐2 inhibitors and as analgesic and anti‐inflammatory agents. Among the synthesized compounds, 6‐benzyl‐2‐methylpyridazin‐3(2H)‐one (4a), 6‐benzoyl‐2‐propylpyridazin‐3(2H)‐one (8b), and 6‐(hydroxy(phenyl)methyl)‐2‐methylpyridazin‐3(2H)‐one (9a) displayed the highest COX‐2 selectivity indices of 96, 99, and 98, respectively, and analgesic efficacies of 47%, 46%, and 45% protection, respectively. Also, compounds 4a, 8b, and 9a showed anti‐inflammatory activities of 65%, 60%, and 62% inhibition of edema, respectively, at a dose of 10 mg/kg, which is higher than that of diclofenac (58% inhibition of edema).
中文翻译:

一些新型 6-取代 2-烷基哒嗪-3(2H)-酮类抗炎镇痛药的合成与生物筛选
通过将 3,6-二氯哒嗪与苄基氰钠盐缩合,然后水解并与卤代烷偶联,合成了一些 2-烷基 6-取代哒嗪-3(2H)-酮的新型衍生物。合成的化合物被筛选为环氧合酶 (COX)-1/COX-2 抑制剂以及镇痛和抗炎剂。在合成的化合物中,6-苄基-2-甲基哒嗪-3(2H)-酮(4a)、6-苯甲酰基-2-丙基哒嗪-3(2H)-酮(8b)和6-(羟基(苯基)甲基) )-2-甲基哒嗪-3(2H)-one (9a) 的 COX-2 选择性指数最高,分别为 96、99 和 98,镇痛功效分别为 47%、46% 和 45%。此外,化合物 4a、8b 和 9a 在 10 mg/kg 的剂量下分别显示出 65%、60% 和 62% 的抗炎活性抑制水肿,
更新日期:2020-01-15
中文翻译:

一些新型 6-取代 2-烷基哒嗪-3(2H)-酮类抗炎镇痛药的合成与生物筛选
通过将 3,6-二氯哒嗪与苄基氰钠盐缩合,然后水解并与卤代烷偶联,合成了一些 2-烷基 6-取代哒嗪-3(2H)-酮的新型衍生物。合成的化合物被筛选为环氧合酶 (COX)-1/COX-2 抑制剂以及镇痛和抗炎剂。在合成的化合物中,6-苄基-2-甲基哒嗪-3(2H)-酮(4a)、6-苯甲酰基-2-丙基哒嗪-3(2H)-酮(8b)和6-(羟基(苯基)甲基) )-2-甲基哒嗪-3(2H)-one (9a) 的 COX-2 选择性指数最高,分别为 96、99 和 98,镇痛功效分别为 47%、46% 和 45%。此外,化合物 4a、8b 和 9a 在 10 mg/kg 的剂量下分别显示出 65%、60% 和 62% 的抗炎活性抑制水肿,



























 京公网安备 11010802027423号
京公网安备 11010802027423号