当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Spirorhodanine-Pyran Derivatives via Organocatalytic [3 + 3] Annulation Reactions between Pyrazolones and Rhodanine-Derived Ketoesters.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.orglett.9b04571 Dong-Sheng Ji 1 , Yong-Chun Luo 1 , Xiu-Qin Hu 1 , Peng-Fei Xu 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.orglett.9b04571 Dong-Sheng Ji 1 , Yong-Chun Luo 1 , Xiu-Qin Hu 1 , Peng-Fei Xu 1
Affiliation
|
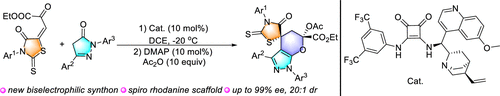
A series of novel biselectrophilic β,γ-unsaturated α-ketoesters were designed and synthesized from rhodanine. Under the catalysis of chiral squaramides, the enantioselective [3 + 3] annulation reaction of these novel ketoesters with pyrazolones was developed. This reaction offers an efficient method for the synthesis of chiral 2'-thioxo-5,6-dihydrospiro[pyrano[2,3-c]pyrazole-4,5'-thiazolidin]-4'-ones.
中文翻译:

通过吡唑啉酮与若丹宁衍生的酮酸酯之间的有机催化[3 + 3]环合反应对映体合成吡咯烷丹宁-吡喃衍生物。
由若丹宁设计合成了一系列新型的双亲电子的β,γ-不饱和α-酮酸酯。在手性方酰胺的催化下,开发了这些新型酮酯与吡唑啉酮的对映体选择性[3 + 3]环化反应。该反应提供了一种有效的手性2'-thioxo-5,6-dihydrospiro [pyrano [2,3-c] pyrazole-4,5'-thiazolidin] -4'-ones的合成方法。
更新日期:2020-01-15
中文翻译:

通过吡唑啉酮与若丹宁衍生的酮酸酯之间的有机催化[3 + 3]环合反应对映体合成吡咯烷丹宁-吡喃衍生物。
由若丹宁设计合成了一系列新型的双亲电子的β,γ-不饱和α-酮酸酯。在手性方酰胺的催化下,开发了这些新型酮酯与吡唑啉酮的对映体选择性[3 + 3]环化反应。该反应提供了一种有效的手性2'-thioxo-5,6-dihydrospiro [pyrano [2,3-c] pyrazole-4,5'-thiazolidin] -4'-ones的合成方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号