当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of molecular oxygen diffusion in a hypoxia sensing prolyl hydroxylase using multiscale simulation.
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-15 , DOI: 10.1021/jacs.9b09236 Carmen Domene 1, 2, 3 , Christian Jorgensen 2 , Christopher J Schofield 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-15 , DOI: 10.1021/jacs.9b09236 Carmen Domene 1, 2, 3 , Christian Jorgensen 2 , Christopher J Schofield 1
Affiliation
|
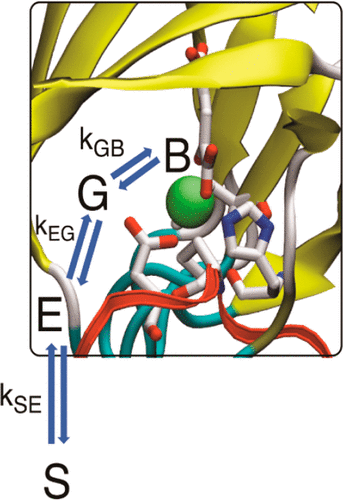
The chronic response of animals to hypoxia is mediated by the αβ-heterodimeric hypoxia-inducible transcription factors (α,β-HIFs) which upregulate the expression of sets of genes that work to ameliorate the effects of limiting dioxygen. The HIF prolyl hydroxylase domain enzymes (PHDs) are Fe(II) and 2-oxoglutarate dependent oxygenases that act as hypoxia sensing components of the HIF system: prolyl-hydroxylation signals for dioxgen availability dependent HIF-α degradation via the ubiquitin proteasome system. The unusual kinetic properties of the PHDs, in particular a high Km for dioxygen and slow reaction with dioxygen are proposed to enable their hypoxia sensing role. An understanding of how dioxygen is delivered to, and binds at, the active site of the PHDs is important for the development of a chemical understanding of the hypoxic response. We employed a combined multiscale approach involving classical atomistic equilibrium and non-equilibrium MD simulations combined with QM/MM trajectories to investigate dioxygen diffusion to, and binding at, the active site in the PHD2.Fe(II).2OG.HIF substrate complex; PHD2 is the most important of the three human PHDs. The transport of dioxygen to the active site is described; dioxygen transport follows a single well-defined hydrophobic tunnel, formed from both enzyme and substrate elements to reach the PHD2 active site. The results provide estimates for rate constants that define a diffusion-reaction model for dioxygen:PHD2 interactions; in combination with reported biophysical analyses they provide chemical insight into the basis of the slow reaction of PHD2 with dioxygen. They imply that the reversible binding of dioxygen is central to the hypoxia sensing capacity of the PHDs and that different PHD HIF-α substrate combinations might have different dioxygen sensitivity profiles. The extent of HIF-α substrate prolyl hydroxylation, which signals for subsequent HIF-α degradation is thus a manifestation of the equilibrium between dioxygen in bulk solution and dioxygen bound to the PHD2.Fe.2OG.HIF-α substrate complex.
中文翻译:

使用多尺度模拟在缺氧传感脯氨酰羟化酶中分子氧扩散的机制。
动物对缺氧的慢性反应是由 αβ-异二聚体缺氧诱导转录因子 (α,β-HIFs) 介导的,该转录因子上调一系列基因的表达,以改善限制分子氧的影响。HIF 脯氨酰羟化酶域酶 (PHD) 是 Fe(II) 和 2-酮戊二酸依赖性加氧酶,它们充当 HIF 系统的缺氧传感组件:脯氨酰羟基化信号用于通过泛素蛋白酶体系统进行双氧可用性依赖的 HIF-α 降解。提出了 PHD 不寻常的动力学特性,特别是分子氧的高 Km 和与分子氧的缓慢反应,以实现它们的缺氧传感作用。了解分子氧如何传递到 PHD 的活性位点并与其结合对于发展对缺氧反应的化学理解很重要。我们采用了一种组合多尺度方法,包括经典原子平衡和非平衡 MD 模拟与 QM/MM 轨迹相结合,以研究分子氧扩散到 PHD2.Fe(II).2OG.HIF 底物复合物中的活性位点并与其结合;PHD2 是三个人类 PHD 中最重要的。描述了分子氧向活性位点的运输;双氧运输遵循一个明确的疏水隧道,由酶和底物元素形成,到达 PHD2 活性位点。结果提供了速率常数的估计值,这些常数定义了分子氧:PHD2 相互作用的扩散反应模型;结合报告的生物物理分析,它们提供了对 PHD2 与分子氧缓慢反应基础的化学洞察力。它们意味着分子氧的可逆结合是 PHD 缺氧感知能力的核心,并且不同的 PHD HIF-α 底物组合可能具有不同的分子氧敏感性特征。HIF-α 底物脯氨酰羟基化的程度,即随后 HIF-α 降解的信号,因此是本体溶液中分子氧与结合到 PHD2.Fe.2OG.HIF-α 底物复合物的分子氧之间平衡的表现。
更新日期:2020-01-15
中文翻译:

使用多尺度模拟在缺氧传感脯氨酰羟化酶中分子氧扩散的机制。
动物对缺氧的慢性反应是由 αβ-异二聚体缺氧诱导转录因子 (α,β-HIFs) 介导的,该转录因子上调一系列基因的表达,以改善限制分子氧的影响。HIF 脯氨酰羟化酶域酶 (PHD) 是 Fe(II) 和 2-酮戊二酸依赖性加氧酶,它们充当 HIF 系统的缺氧传感组件:脯氨酰羟基化信号用于通过泛素蛋白酶体系统进行双氧可用性依赖的 HIF-α 降解。提出了 PHD 不寻常的动力学特性,特别是分子氧的高 Km 和与分子氧的缓慢反应,以实现它们的缺氧传感作用。了解分子氧如何传递到 PHD 的活性位点并与其结合对于发展对缺氧反应的化学理解很重要。我们采用了一种组合多尺度方法,包括经典原子平衡和非平衡 MD 模拟与 QM/MM 轨迹相结合,以研究分子氧扩散到 PHD2.Fe(II).2OG.HIF 底物复合物中的活性位点并与其结合;PHD2 是三个人类 PHD 中最重要的。描述了分子氧向活性位点的运输;双氧运输遵循一个明确的疏水隧道,由酶和底物元素形成,到达 PHD2 活性位点。结果提供了速率常数的估计值,这些常数定义了分子氧:PHD2 相互作用的扩散反应模型;结合报告的生物物理分析,它们提供了对 PHD2 与分子氧缓慢反应基础的化学洞察力。它们意味着分子氧的可逆结合是 PHD 缺氧感知能力的核心,并且不同的 PHD HIF-α 底物组合可能具有不同的分子氧敏感性特征。HIF-α 底物脯氨酰羟基化的程度,即随后 HIF-α 降解的信号,因此是本体溶液中分子氧与结合到 PHD2.Fe.2OG.HIF-α 底物复合物的分子氧之间平衡的表现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号