当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capture of the Sulfur Monoxide–Hydroxyl Radical Complex (•OH···OS)
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-15 , DOI: 10.1021/jacs.9b12152 Changyun Chen 1 , Bo Lu 1 , Xiaofang Zhao 1 , Weiyu Qian 1 , Jie Liu 1 , Tarek Trabelsi 2 , Joseph S Francisco 2 , Jie Qin 3 , Jun Li 3 , Lina Wang 4 , Xiaoqing Zeng 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-15 , DOI: 10.1021/jacs.9b12152 Changyun Chen 1 , Bo Lu 1 , Xiaofang Zhao 1 , Weiyu Qian 1 , Jie Liu 1 , Tarek Trabelsi 2 , Joseph S Francisco 2 , Jie Qin 3 , Jun Li 3 , Lina Wang 4 , Xiaoqing Zeng 1, 4
Affiliation
|
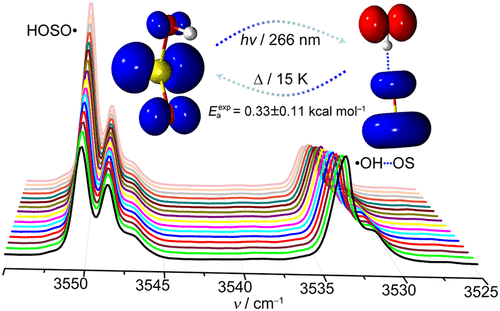
The elusive hydrogen-bonded sulfur monoxide-hydroxyl radical complex (•OH···OS), a missing intermediate in the atmospheric chemistry of sulfur dioxide, has been generated in the 266 nm laser photolysis of the atmospheric relevant sulfinyl radical HOSO• in cryogenic Ar-matrices. In addition to the IR spectroscopic characterization with deuteration, its thermal conversion to HOSO• with an activation barrier of 0.330.11 kcal mol-1 (cal. 0.32 kcal mol-1, CCSD(T)-F12a/AVTZ) in the temperature range of 15.0-21.0 K and a H/D kinetic isotope effect (KIE) of 2.4 at 16.0 K have been observed.
中文翻译:

一氧化硫-羟基自由基复合物(•OH···OS)的捕获
难以捉摸的氢键一氧化硫-羟基自由基复合物 (•OH···OS) 是二氧化硫大气化学中缺失的中间体,它是在低温下大气相关亚磺酰基自由基 HOSO• 的 266 nm 激光光解中产生的。矩阵。除了用氘进行红外光谱表征外,其热转化为 HOSO• 的活化势垒为 0.330.11 kcal mol-1 (cal. 0.32 kcal mol-1, CCSD(T)-F12a/AVTZ) 在温度范围内已观察到 15.0-21.0 K 和 2.4 的 H/D 动力学同位素效应 (KIE) 在 16.0 K。
更新日期:2020-01-15
中文翻译:

一氧化硫-羟基自由基复合物(•OH···OS)的捕获
难以捉摸的氢键一氧化硫-羟基自由基复合物 (•OH···OS) 是二氧化硫大气化学中缺失的中间体,它是在低温下大气相关亚磺酰基自由基 HOSO• 的 266 nm 激光光解中产生的。矩阵。除了用氘进行红外光谱表征外,其热转化为 HOSO• 的活化势垒为 0.330.11 kcal mol-1 (cal. 0.32 kcal mol-1, CCSD(T)-F12a/AVTZ) 在温度范围内已观察到 15.0-21.0 K 和 2.4 的 H/D 动力学同位素效应 (KIE) 在 16.0 K。











































 京公网安备 11010802027423号
京公网安备 11010802027423号