当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ketone Synthesis from Benzyldiboronates and Esters: Leveraging α-Borylcarbanions for Carbon-Carbon Bond Formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-15 , DOI: 10.1021/jacs.9b11944 Boran Lee 1 , Paul J Chirik 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-15 , DOI: 10.1021/jacs.9b11944 Boran Lee 1 , Paul J Chirik 1
Affiliation
|
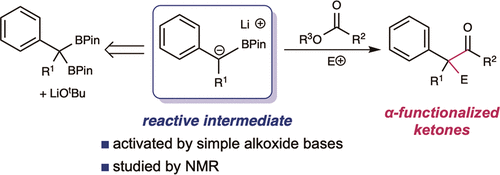
An alkoxide-promoted method for the synthesis of ketones from readily available esters and benzyldiboronates is described. The synthetic method is compatible with a host of sterically differentiated alkyl groups, alkenes, acidic protons, amides and aryl rings having common organic functional groups. With esters bearing α-stereocenters, high enantiomeric excess was maintained during ketone formation, establishing minimal competing racemization by deprotonation. Monitoring the reaction between benzyldiboronate and LiOtBu in THF at 23 ºC allowed for the identification of products arising from deborylation to form an α-borylcarbanion, deprotonation, and alkoxide addition to form an "-ate" complex. Addition of 4-trifluoromethylbenzoate to this mixture established the α-borylcarbanion as responsible for C-C bond formation and ultimately ketone synthesis. Elucidation of the role of this intermediate leveraged additional bond-forming chemistry and enabled the one-pot synthesis of ketones with α-halogen atoms and quaternary centers with four-different carbon substituents.
中文翻译:

从二硼酸苄酯和酯合成酮:利用 α-硼基碳负离子形成碳-碳键
描述了一种由容易获得的酯和二硼酸苄基酯合成酮的醇盐促进方法。该合成方法与具有共同有机官能团的大量空间差异的烷基、烯烃、酸性质子、酰胺和芳环相容。对于带有 α-立体中心的酯,在酮形成过程中保持高对映体过量,通过去质子化建立最小的竞争性外消旋化。在 23 ℃ 下监测二硼酸苄酯和 LiOtBu 在 THF 中的反应,可以鉴定脱硼酸形成 α-硼基碳负离子、去质子化和醇盐加成形成“-ate”络合物所产生的产物。向该混合物中添加 4-三氟甲基苯甲酸酯,确定 α-硼基碳负离子负责 CC 键的形成和最终的酮合成。该中间体的作用的阐明利用了额外的成键化学,并实现了具有 α-卤素原子和具有四种不同碳取代基的四元中心的酮的一锅合成。
更新日期:2020-01-15
中文翻译:

从二硼酸苄酯和酯合成酮:利用 α-硼基碳负离子形成碳-碳键
描述了一种由容易获得的酯和二硼酸苄基酯合成酮的醇盐促进方法。该合成方法与具有共同有机官能团的大量空间差异的烷基、烯烃、酸性质子、酰胺和芳环相容。对于带有 α-立体中心的酯,在酮形成过程中保持高对映体过量,通过去质子化建立最小的竞争性外消旋化。在 23 ℃ 下监测二硼酸苄酯和 LiOtBu 在 THF 中的反应,可以鉴定脱硼酸形成 α-硼基碳负离子、去质子化和醇盐加成形成“-ate”络合物所产生的产物。向该混合物中添加 4-三氟甲基苯甲酸酯,确定 α-硼基碳负离子负责 CC 键的形成和最终的酮合成。该中间体的作用的阐明利用了额外的成键化学,并实现了具有 α-卤素原子和具有四种不同碳取代基的四元中心的酮的一锅合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号