当前位置:
X-MOL 学术
›
Commun. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural analysis of lecithin:cholesterol acyltransferase bound to high density lipoprotein particles.
Communications Biology ( IF 5.2 ) Pub Date : 2020-01-15 , DOI: 10.1038/s42003-019-0749-z Kelly A Manthei 1 , Dhabaleswar Patra 2 , Christopher J Wilson 3 , Maria V Fawaz 4 , Lolita Piersimoni 5 , Jenny Capua Shenkar 6 , Wenmin Yuan 6 , Philip C Andrews 5 , John R Engen 3 , Anna Schwendeman 6 , Melanie D Ohi 7 , John J G Tesmer 2
Communications Biology ( IF 5.2 ) Pub Date : 2020-01-15 , DOI: 10.1038/s42003-019-0749-z Kelly A Manthei 1 , Dhabaleswar Patra 2 , Christopher J Wilson 3 , Maria V Fawaz 4 , Lolita Piersimoni 5 , Jenny Capua Shenkar 6 , Wenmin Yuan 6 , Philip C Andrews 5 , John R Engen 3 , Anna Schwendeman 6 , Melanie D Ohi 7 , John J G Tesmer 2
Affiliation
|
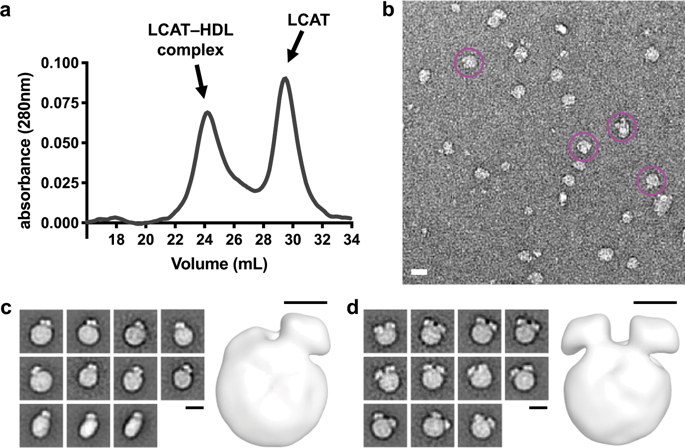
Lecithin:cholesterol acyltransferase (LCAT) catalyzes a critical step of reverse cholesterol transport by esterifying cholesterol in high density lipoprotein (HDL) particles. LCAT is activated by apolipoprotein A-I (ApoA-I), which forms a double belt around HDL, however the manner in which LCAT engages its lipidic substrates and ApoA-I in HDL is poorly understood. Here, we used negative stain electron microscopy, crosslinking, and hydrogen-deuterium exchange studies to refine the molecular details of the LCAT-HDL complex. Our data are consistent with LCAT preferentially binding to the edge of discoidal HDL near the boundary between helix 5 and 6 of ApoA-I in a manner that creates a path from the lipid bilayer to the active site of LCAT. Our results provide not only an explanation why LCAT activity diminishes as HDL particles mature, but also direct support for the anti-parallel double belt model of HDL, with LCAT binding preferentially to the helix 4/6 region.
中文翻译:

卵磷脂的结构分析:与高密度脂蛋白颗粒结合的胆固醇酰基转移酶。
卵磷脂:胆固醇酰基转移酶 (LCAT) 通过酯化高密度脂蛋白 (HDL) 颗粒中的胆固醇,催化反向胆固醇转运的关键步骤。LCAT 由载脂蛋白 AI (ApoA-I) 激活,它在 HDL 周围形成双带,但是 LCAT 与脂质底物和 ApoA-I 在 HDL 中的结合方式知之甚少。在这里,我们使用负染色电子显微镜、交联和氢-氘交换研究来改进 LCAT-HDL 复合物的分子细节。我们的数据与 LCAT 优先结合 ApoA-I 的螺旋 5 和 6 之间的边界附近的盘状 HDL 边缘一致,这种方式创建了从脂质双层到 LCAT 活性位点的路径。我们的结果不仅解释了为什么 LCAT 活性随着 HDL 颗粒成熟而减弱,
更新日期:2020-01-15
中文翻译:

卵磷脂的结构分析:与高密度脂蛋白颗粒结合的胆固醇酰基转移酶。
卵磷脂:胆固醇酰基转移酶 (LCAT) 通过酯化高密度脂蛋白 (HDL) 颗粒中的胆固醇,催化反向胆固醇转运的关键步骤。LCAT 由载脂蛋白 AI (ApoA-I) 激活,它在 HDL 周围形成双带,但是 LCAT 与脂质底物和 ApoA-I 在 HDL 中的结合方式知之甚少。在这里,我们使用负染色电子显微镜、交联和氢-氘交换研究来改进 LCAT-HDL 复合物的分子细节。我们的数据与 LCAT 优先结合 ApoA-I 的螺旋 5 和 6 之间的边界附近的盘状 HDL 边缘一致,这种方式创建了从脂质双层到 LCAT 活性位点的路径。我们的结果不仅解释了为什么 LCAT 活性随着 HDL 颗粒成熟而减弱,











































 京公网安备 11010802027423号
京公网安备 11010802027423号