当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Boron Substitution on Oxide-Ion Conduction in c-Axis-Oriented Apatite-Type Lanthanum Silicate
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jpcc.9b11454 Shingo Ide 1, 2 , Hiroki Takahashi 2 , Isamu Yashima 2 , Koichi Suematsu 3 , Ken Watanabe 3 , Kengo Shimanoe 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jpcc.9b11454 Shingo Ide 1, 2 , Hiroki Takahashi 2 , Isamu Yashima 2 , Koichi Suematsu 3 , Ken Watanabe 3 , Kengo Shimanoe 3
Affiliation
|
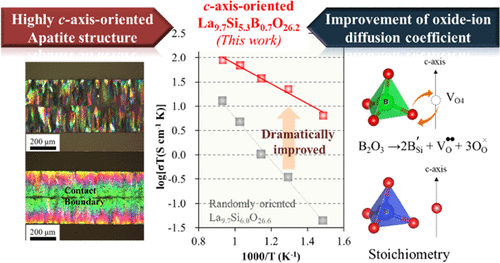
Apatite-type lanthanum silicate (LSO) is a material with high oxide-ion conductivity in the low- and intermediate-temperature range (573–873 K) and is, therefore, a promising solid electrolyte for low-temperature applications such as solid oxide fuel cells and oxygen sensors. Herein, the effect of B substitution at the Si site in a c-axis-oriented apatite-type lanthanum silicate (La9.7Si5.3B0.7O26.2, c-LSBO) polycrystal on oxide-ion conduction is investigated. A highly c-axis-oriented LSBO polycrystal is fabricated by a vapor–solid reaction in which a dense La2SiO5 disk is heated in B2O3 vapor at ≥1673 K. The oxide-ion conductivity of c-LSBO reaches 16 mS cm–1 at 678 K with an activation energy of 0.4 eV. The obtained oxide-ion conductivity of c-LSBO is approximately 190 times higher than that of yttria-stabilized zirconia and 5.8 times higher than that of the polycrystalline c-axis-oriented nondoped lanthanum silicate. Based on 11B nuclear magnetic resonance measurements, B is located at the SiO4 site as BO4, suggesting the formation of an oxygen vacancy at the O4 site located along the c-axis due to charge compensation. In addition, molecular dynamics simulations indicate that the oxide-ion diffusion coefficient of the B-doped LSO is higher than that of the nondoped LSO. The high oxide-ion conductivity of c-LSBO is likely attributable to the formation of an oxygen vacancy at the O4 site by B doping, which has a lower valency than Si. Therefore, c-LSBO is a promising candidate as a solid electrolyte in electrochemical devices operating at low and moderately high temperatures.
中文翻译:

硼取代对c轴取向磷灰石型硅酸镧中氧化物离子导电的影响
磷灰石型硅酸镧(LSO)是在低温和中温范围(573–873 K)中具有高氧化物离子电导率的材料,因此,它是用于低温应用的固体电解质,例如固体氧化物燃料电池和氧气传感器。在此,研究了在c轴取向的磷灰石型硅酸镧(La 9.7 Si 5.3 B 0.7 O 26.2,c -LSBO)多晶体中,Si位点处的B取代对氧离子传导的影响。通过汽固反应制备高c轴取向的LSBO多晶,其中在B 2中加热致密的La 2 SiO 5圆盘ø 3蒸气在≥1673K.的氧化物离子传导性C- LSBO达到16毫秒cm的-1在678 K的为0.4eV的活化能。所获得的c -LSBO的氧化物离子电导率大约是氧化钇稳定的氧化锆的190倍,是多晶c轴取向的非掺杂硅酸镧的5.8倍。根据11个B核磁共振测量,B位于SiO 4位置,称为BO 4,表明沿c的O4位置形成了氧空位。轴由于电荷补偿。另外,分子动力学模拟表明,B掺杂的LSO的氧化物离子扩散系数高于非掺杂的LSO的氧化物扩散系数。c -LSBO的高氧化物离子电导率很可能归因于通过B掺杂在O4处形成氧空位,其价价比Si低。因此,c -LSBO是在低温和中等高温下运行的电化学装置中作为固体电解质的有前途的候选者。
更新日期:2020-01-26
中文翻译:

硼取代对c轴取向磷灰石型硅酸镧中氧化物离子导电的影响
磷灰石型硅酸镧(LSO)是在低温和中温范围(573–873 K)中具有高氧化物离子电导率的材料,因此,它是用于低温应用的固体电解质,例如固体氧化物燃料电池和氧气传感器。在此,研究了在c轴取向的磷灰石型硅酸镧(La 9.7 Si 5.3 B 0.7 O 26.2,c -LSBO)多晶体中,Si位点处的B取代对氧离子传导的影响。通过汽固反应制备高c轴取向的LSBO多晶,其中在B 2中加热致密的La 2 SiO 5圆盘ø 3蒸气在≥1673K.的氧化物离子传导性C- LSBO达到16毫秒cm的-1在678 K的为0.4eV的活化能。所获得的c -LSBO的氧化物离子电导率大约是氧化钇稳定的氧化锆的190倍,是多晶c轴取向的非掺杂硅酸镧的5.8倍。根据11个B核磁共振测量,B位于SiO 4位置,称为BO 4,表明沿c的O4位置形成了氧空位。轴由于电荷补偿。另外,分子动力学模拟表明,B掺杂的LSO的氧化物离子扩散系数高于非掺杂的LSO的氧化物扩散系数。c -LSBO的高氧化物离子电导率很可能归因于通过B掺杂在O4处形成氧空位,其价价比Si低。因此,c -LSBO是在低温和中等高温下运行的电化学装置中作为固体电解质的有前途的候选者。










































 京公网安备 11010802027423号
京公网安备 11010802027423号