当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thyroid hormone inhibits hepatocellular carcinoma progression via induction of differentiation and metabolic reprogramming
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jhep.2019.12.018 Marta Anna Kowalik 1 , Elisabetta Puliga 2 , Lavinia Cabras 1 , Pia Sulas 1 , Annalisa Petrelli 3 , Andrea Perra 1 , Giovanna Maria Ledda-Columbano 1 , Andrea Morandi 4 , Simone Merlin 5 , Claudia Orrù 2 , Carlos Sanchez-Martin 6 , Francesca Fornari 7 , Laura Gramantieri 7 , Matteo Parri 4 , Andrea Rasola 6 , Sara Erika Bellomo 3 , Carlos Sebastian 3 , Antonia Follenzi 5 , Silvia Giordano 8 , Amedeo Columbano 1
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.jhep.2019.12.018 Marta Anna Kowalik 1 , Elisabetta Puliga 2 , Lavinia Cabras 1 , Pia Sulas 1 , Annalisa Petrelli 3 , Andrea Perra 1 , Giovanna Maria Ledda-Columbano 1 , Andrea Morandi 4 , Simone Merlin 5 , Claudia Orrù 2 , Carlos Sanchez-Martin 6 , Francesca Fornari 7 , Laura Gramantieri 7 , Matteo Parri 4 , Andrea Rasola 6 , Sara Erika Bellomo 3 , Carlos Sebastian 3 , Antonia Follenzi 5 , Silvia Giordano 8 , Amedeo Columbano 1
Affiliation
|
BACKGROUND & AIMS
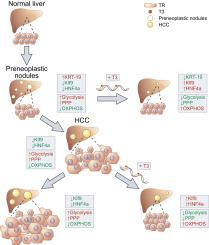
The limited therapeutic options available for hepatocellular carcinoma (HCC) make mandatory to find alternative effective treatments for this tumor. Based on the recent finding that systemic or local hypothyroidism is associated with HCC development in humans and rodents, we investigated whether the thyroid hormone triiodothyronine (T3) could inhibit the progression of hepatocellular carcinomas (HCCs). METHODS
Different rat and mouse models of hepatocarcinogenesis were investigated. The effect of T3 on tumorigenesis and metabolism/differentiation was evaluated by transcriptomic analysis, quantitative-PCR, immunohistochemistry, and enzymatic assay. RESULTS
A short treatment with T3 caused a shift of global expression profile of the most aggressive preneoplastic nodules towards that of normal liver. This genomic reprogramming preceded the disappearance of nodules and involved reprogramming of metabolic genes as well as pro-differentiating transcription factors, including Kruppel-like factor 9 (Klf9), a target of the thyroid hormone receptor β (TRβ). Treatment of HCCs-bearing rats with T3 strongly reduced the number and burden of HCCs. Reactivation of a local T3/TRβ axis, switch from Warburg to oxidative metabolism and loss of markers of poorly differentiated hepatocytes accompanied HCC reduction. This effect persisted one month after T3 withdrawal suggesting a long-lasting effect of the hormone. The antitumorigenic effect of T3 was further supported by its inhibitory activity on cell growth and tumorigenic ability of human HCC cell lines. CONCLUSIONS
Collectively, these findings suggest that re-activation of the T3/TRβ axis induces differentiation of neoplastic cells towards a more benign phenotype and that T3 or its analogues, particularly agonists of the TRβ, can represent useful tools in HCC therapy.
中文翻译:

甲状腺激素通过诱导分化和代谢重编程抑制肝细胞癌进展
背景和目的 肝细胞癌 (HCC) 可用的有限治疗选择使得必须为这种肿瘤寻找替代的有效治疗方法。基于最近发现全身或局部甲状腺功能减退与人类和啮齿动物的 HCC 发展有关,我们研究了甲状腺激素三碘甲腺原氨酸 (T3) 是否可以抑制肝细胞癌 (HCC) 的进展。方法研究了不同的大鼠和小鼠肝癌发生模型。T3 对肿瘤发生和代谢/分化的影响通过转录组学分析、定量 PCR、免疫组织化学和酶分析进行评估。结果 T3 的短期治疗导致最具侵袭性的肿瘤前结节的整体表达谱向正常肝脏转变。这种基因组重编程在结节消失之前,涉及代谢基因的重编程以及促分化转录因子,包括 Kruppel 样因子 9 (Klf9),甲状腺激素受体 β (TRβ) 的靶标。用 T3 治疗携带 HCC 的大鼠大大减少了 HCC 的数量和负担。局部 T3/TRβ 轴的重新激活、从 Warburg 转变为氧化代谢以及低分化肝细胞标志物的丢失伴随着 HCC 的减少。这种效应在 T3 戒断后持续一个月,表明激素的长期作用。T3 的抗肿瘤作用得到其对人 HCC 细胞系的细胞生长和致瘤能力的抑制活性的进一步支持。结论 总的来说,
更新日期:2020-06-01
中文翻译:

甲状腺激素通过诱导分化和代谢重编程抑制肝细胞癌进展
背景和目的 肝细胞癌 (HCC) 可用的有限治疗选择使得必须为这种肿瘤寻找替代的有效治疗方法。基于最近发现全身或局部甲状腺功能减退与人类和啮齿动物的 HCC 发展有关,我们研究了甲状腺激素三碘甲腺原氨酸 (T3) 是否可以抑制肝细胞癌 (HCC) 的进展。方法研究了不同的大鼠和小鼠肝癌发生模型。T3 对肿瘤发生和代谢/分化的影响通过转录组学分析、定量 PCR、免疫组织化学和酶分析进行评估。结果 T3 的短期治疗导致最具侵袭性的肿瘤前结节的整体表达谱向正常肝脏转变。这种基因组重编程在结节消失之前,涉及代谢基因的重编程以及促分化转录因子,包括 Kruppel 样因子 9 (Klf9),甲状腺激素受体 β (TRβ) 的靶标。用 T3 治疗携带 HCC 的大鼠大大减少了 HCC 的数量和负担。局部 T3/TRβ 轴的重新激活、从 Warburg 转变为氧化代谢以及低分化肝细胞标志物的丢失伴随着 HCC 的减少。这种效应在 T3 戒断后持续一个月,表明激素的长期作用。T3 的抗肿瘤作用得到其对人 HCC 细胞系的细胞生长和致瘤能力的抑制活性的进一步支持。结论 总的来说,











































 京公网安备 11010802027423号
京公网安备 11010802027423号