当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Monitoring biomolecular interaction between folic acid and bovine serum albumin.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.saa.2020.118074 Claudia G Chilom 1 , Melinda David 2 , Monica Florescu 2
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.saa.2020.118074 Claudia G Chilom 1 , Melinda David 2 , Monica Florescu 2
Affiliation
|
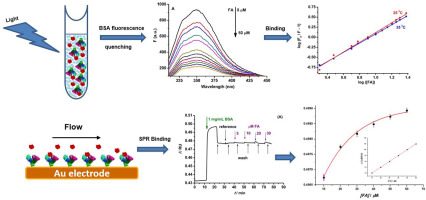
Folic acid is a bioactive food component whose deficiency can lead to a variety of health problems, while a high intake of folic acid can reduce the cytotoxicity of natural killer cells. The binding mechanism of folic acid to free bovine serum albumin (BSA) was studied using fluorescence, while the biomolecular interaction between confined-BSA and free folic acid was assessed by electrochemical methods and surface plasmon resonance. The fluorescence quenching mechanism of BSA by folic acid was found to have a static character. The thermodynamic parameters of the interaction were determined and indicated a spontaneous exothermic process with a binding constant of 8.72 × 104 M-1 at 25 °C. Confinement of BSA to gold surfaces occurred through different immobilization methods (static and hydrodynamic), inducing conformational changes, which influenced the orientation of BSA molecules binding sites towards free folic acid. The apparent binding constant using electrochemical methods (voltammetry and impedance spectroscopy) was only 5 times higher (41 and 37 × 104 M-1) compared to BSA free in solution, while for surface plasmon resonance, where the hydrodynamic immobilization method was used, the value was much higher (19 × 106 M-1). This work gives also an insight on the interaction of BSA with gold substrates, surface plasmon resonance enabling the calculation of the adsorbed amount. The obtained results help understanding the specific interaction between free and confined BSA with free folic acid.
中文翻译:

监测叶酸和牛血清白蛋白之间的生物分子相互作用。
叶酸是一种生物活性食品成分,其缺乏会导致多种健康问题,而高叶酸摄入量则可以减少自然杀伤细胞的细胞毒性。利用荧光研究了叶酸与游离牛血清白蛋白(BSA)的结合机理,同时通过电化学方法和表面等离子体共振评价了封闭的BSA与游离叶酸之间的生物分子相互作用。发现叶酸对BSA的荧光猝灭机制具有静态特性。确定了相互作用的热力学参数,并表明在25°C下结合常数为8.72×104 M-1的自发放热过程。通过不同的固定方法(静态和流体动力学)将BSA限制在金表面,从而引起构象变化,这影响了BSA分子结合位点朝向游离叶酸的方向。与溶液中不含BSA的溶液相比,使用电化学方法(伏安法和阻抗谱法)的表观结合常数(41和37×104 M-1)仅高5倍,而对于表面等离振子共振(使用流体动力固定化方法),值更高(19×106 M-1)。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。与溶液中不含BSA的溶液相比,使用电化学方法(伏安法和阻抗谱)的表观结合常数(41和37×104 M-1)仅高5倍,而对于表面等离振子共振(使用流体动力固定化方法),值更高(19×106 M-1)。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。与溶液中不含BSA的溶液相比,使用电化学方法(伏安法和阻抗谱法)的表观结合常数(41和37×104 M-1)仅高5倍,而对于表面等离振子共振(使用流体动力固定化方法),值更高(19×106 M-1)。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。
更新日期:2020-01-15
中文翻译:

监测叶酸和牛血清白蛋白之间的生物分子相互作用。
叶酸是一种生物活性食品成分,其缺乏会导致多种健康问题,而高叶酸摄入量则可以减少自然杀伤细胞的细胞毒性。利用荧光研究了叶酸与游离牛血清白蛋白(BSA)的结合机理,同时通过电化学方法和表面等离子体共振评价了封闭的BSA与游离叶酸之间的生物分子相互作用。发现叶酸对BSA的荧光猝灭机制具有静态特性。确定了相互作用的热力学参数,并表明在25°C下结合常数为8.72×104 M-1的自发放热过程。通过不同的固定方法(静态和流体动力学)将BSA限制在金表面,从而引起构象变化,这影响了BSA分子结合位点朝向游离叶酸的方向。与溶液中不含BSA的溶液相比,使用电化学方法(伏安法和阻抗谱法)的表观结合常数(41和37×104 M-1)仅高5倍,而对于表面等离振子共振(使用流体动力固定化方法),值更高(19×106 M-1)。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。与溶液中不含BSA的溶液相比,使用电化学方法(伏安法和阻抗谱)的表观结合常数(41和37×104 M-1)仅高5倍,而对于表面等离振子共振(使用流体动力固定化方法),值更高(19×106 M-1)。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。与溶液中不含BSA的溶液相比,使用电化学方法(伏安法和阻抗谱法)的表观结合常数(41和37×104 M-1)仅高5倍,而对于表面等离振子共振(使用流体动力固定化方法),值更高(19×106 M-1)。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。这项工作还对BSA与金基质的相互作用,表面等离振子共振提供了见解,从而可以计算吸附量。获得的结果有助于理解游离的和封闭的BSA与游离叶酸之间的具体相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号