当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(Electro-)chemical Splitting of Dinitrogen with a Rhenium Pincer Complex
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-01-31 , DOI: 10.1002/ejic.201901278 Richt S van Alten 1 , Florian Wätjen 1 , Serhiy Demeshko 1 , Alexander J M Miller 2 , Christian Würtele 1 , Inke Siewert 1, 3 , Sven Schneider 1, 3
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-01-31 , DOI: 10.1002/ejic.201901278 Richt S van Alten 1 , Florian Wätjen 1 , Serhiy Demeshko 1 , Alexander J M Miller 2 , Christian Würtele 1 , Inke Siewert 1, 3 , Sven Schneider 1, 3
Affiliation
|
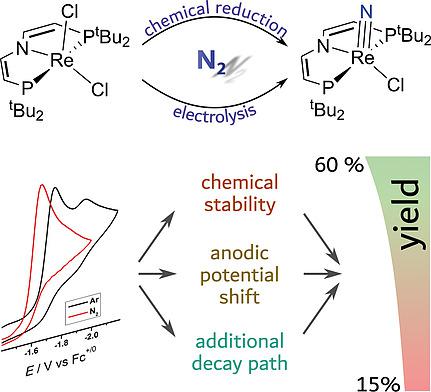
The splitting of N2 into well‐defined terminal nitride complexes is a key reaction for nitrogen fixation at ambient conditions. In continuation of our previous work on rhenium pincer mediated N2 splitting, nitrogen activation and cleavage upon (electro)chemical reduction of [ReCl2(L2)] {L2 = N(CHCHPtBu2)2 –} is reported. The electrochemical characterization of [ReCl2(L2)] and comparison with our previously reported platform [ReCl2(L1)] {L1 = N(CH2CH2PtBu2)2 –} provides mechanistic insight to rationalize the dependence of nitride yield on the reductant. Furthermore, the reactivity of N2 derived nitride complex [Re(N)Cl(L2)] with electrophiles is presented.
中文翻译:

二氮与铼钳络合物的(电化学)化学分裂
N2 分裂成明确定义的末端氮化物复合物是环境条件下固氮的关键反应。继续我们之前关于铼钳介导的 N2 分裂、氮活化和裂解 [ReCl2(L2)] {L2 = N(CHCHPtBu2)2 –} 的(电)化学还原。[ReCl2(L2)] 的电化学表征以及与我们之前报道的平台 [ReCl2(L1)] {L1 = N(CH2CH2PtBu2)2 –} 的比较提供了机械洞察力,以合理化氮化物产率对还原剂的依赖性。此外,还介绍了 N2 衍生的氮化物复合物 [Re(N)Cl(L2)] 与亲电子试剂的反应性。
更新日期:2020-01-31
中文翻译:

二氮与铼钳络合物的(电化学)化学分裂
N2 分裂成明确定义的末端氮化物复合物是环境条件下固氮的关键反应。继续我们之前关于铼钳介导的 N2 分裂、氮活化和裂解 [ReCl2(L2)] {L2 = N(CHCHPtBu2)2 –} 的(电)化学还原。[ReCl2(L2)] 的电化学表征以及与我们之前报道的平台 [ReCl2(L1)] {L1 = N(CH2CH2PtBu2)2 –} 的比较提供了机械洞察力,以合理化氮化物产率对还原剂的依赖性。此外,还介绍了 N2 衍生的氮化物复合物 [Re(N)Cl(L2)] 与亲电子试剂的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号