当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Chimeric EccB-MycP Fusion Protein is Functional and a Stable Component of the ESX-5 Type VII Secretion System Membrane Complex.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-14 , DOI: 10.1016/j.jmb.2019.12.040 Vincent J C van Winden 1 , Catalin M Bunduc 2 , Roy Ummels 1 , Wilbert Bitter 3 , Edith N G Houben 2
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-01-14 , DOI: 10.1016/j.jmb.2019.12.040 Vincent J C van Winden 1 , Catalin M Bunduc 2 , Roy Ummels 1 , Wilbert Bitter 3 , Edith N G Houben 2
Affiliation
|
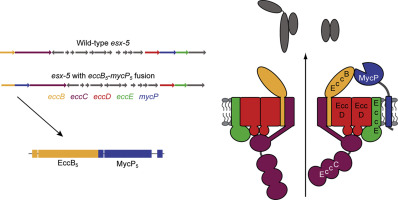
The mycosin protease (MycP) is widely conserved in type VII secretion (T7S) systems throughout Actinobacteria. Within the T7S systems of mycobacteria, also known as the ESX systems, MycP is essential for secretion, which is probably linked to its stabilizing effect on the ESX membrane complex. However, it is unknown how this is mediated, as MycP is not a stable component of this complex. In this study, we set out to create a chimeric fusion protein of EccB5 and MycP5, based on a chimeric gene of eccB and mycP in the T7S locus of Bifidobacterium dentium. We show that this fusion protein is functional and capable of complementing ESX-5 secretion in both an eccB5 and a mycP5 knockout in Mycobacterium marinum. To study the ESX complex containing this fusion protein in more detail, we replaced the original eccB5 and mycP5 of the Mycobacterium xenopi esx-5 locus, reconstituted in Mycobacterium smegmatis, with the chimeric gene. The EccB5-MycP5 fusion construct also restored ESX-5 secretion under these double knockout conditions. Subsequent protein pulldowns on the central complex component EccC5 showed that under these conditions, the EccB5-MycP5 fusion was specifically copurified and a stable component of the ESX-5 complex. Based on our results, we can conclude that MycP5 carries out its essential function in secretion in close proximity to EccB5, indicating that EccB5 is the direct interaction partner of MycP5.
中文翻译:

嵌合的EccB-MycP融合蛋白具有功能,并且是ESX-5型VII分泌系统膜复合物的稳定成分。
在整个放线菌中,霉菌素蛋白酶(MycP)在VII型分泌(T7S)系统中广泛保守。在分枝杆菌的T7S系统(也称为ESX系统)中,MycP是分泌所必需的,这可能与其对ESX膜复合物的稳定作用有关。但是,由于MycP并不是这种复合物的稳定成分,因此如何介导它是未知的。在这项研究中,我们着手在牙本质双歧杆菌T7S基因座中的eccB和mycP嵌合基因的基础上,创建EccB5和MycP5的嵌合融合蛋白。我们显示此融合蛋白是功能性的,并且能够在海分枝杆菌中的eccB5和mycP5基因敲除中补充ESX-5分泌。要更详细地研究包含此融合蛋白的ESX复合物,我们用嵌合基因替换了在耻垢分枝杆菌中重组的异种分枝杆菌esx-5基因座的原始eccB5和mycP5。在这些双重敲除条件下,EccB5-MycP5融合构建体还恢复了ESX-5的分泌。随后在中央复合物组分EccC5上的蛋白质下拉显示,在这些条件下,EccB5-MycP5融合物被特异地共纯化,并且是ESX-5复合物的稳定组分。根据我们的结果,我们可以得出结论,MycP5在与EccB5紧密接近的分泌中执行其基本功能,这表明EccB5是MycP5的直接相互作用伙伴。随后在中央复合物组分EccC5上的蛋白质下拉显示,在这些条件下,EccB5-MycP5融合物被特异地共纯化,并且是ESX-5复合物的稳定组分。根据我们的结果,我们可以得出结论,MycP5在与EccB5紧密接近的分泌中执行其基本功能,这表明EccB5是MycP5的直接相互作用伙伴。随后在中央复合物组分EccC5上的蛋白质下拉显示,在这些条件下,EccB5-MycP5融合物被特异地共纯化,并且是ESX-5复合物的稳定组分。根据我们的结果,我们可以得出结论,MycP5在与EccB5紧密接近的分泌中执行其基本功能,这表明EccB5是MycP5的直接相互作用伙伴。
更新日期:2020-01-15
中文翻译:

嵌合的EccB-MycP融合蛋白具有功能,并且是ESX-5型VII分泌系统膜复合物的稳定成分。
在整个放线菌中,霉菌素蛋白酶(MycP)在VII型分泌(T7S)系统中广泛保守。在分枝杆菌的T7S系统(也称为ESX系统)中,MycP是分泌所必需的,这可能与其对ESX膜复合物的稳定作用有关。但是,由于MycP并不是这种复合物的稳定成分,因此如何介导它是未知的。在这项研究中,我们着手在牙本质双歧杆菌T7S基因座中的eccB和mycP嵌合基因的基础上,创建EccB5和MycP5的嵌合融合蛋白。我们显示此融合蛋白是功能性的,并且能够在海分枝杆菌中的eccB5和mycP5基因敲除中补充ESX-5分泌。要更详细地研究包含此融合蛋白的ESX复合物,我们用嵌合基因替换了在耻垢分枝杆菌中重组的异种分枝杆菌esx-5基因座的原始eccB5和mycP5。在这些双重敲除条件下,EccB5-MycP5融合构建体还恢复了ESX-5的分泌。随后在中央复合物组分EccC5上的蛋白质下拉显示,在这些条件下,EccB5-MycP5融合物被特异地共纯化,并且是ESX-5复合物的稳定组分。根据我们的结果,我们可以得出结论,MycP5在与EccB5紧密接近的分泌中执行其基本功能,这表明EccB5是MycP5的直接相互作用伙伴。随后在中央复合物组分EccC5上的蛋白质下拉显示,在这些条件下,EccB5-MycP5融合物被特异地共纯化,并且是ESX-5复合物的稳定组分。根据我们的结果,我们可以得出结论,MycP5在与EccB5紧密接近的分泌中执行其基本功能,这表明EccB5是MycP5的直接相互作用伙伴。随后在中央复合物组分EccC5上的蛋白质下拉显示,在这些条件下,EccB5-MycP5融合物被特异地共纯化,并且是ESX-5复合物的稳定组分。根据我们的结果,我们可以得出结论,MycP5在与EccB5紧密接近的分泌中执行其基本功能,这表明EccB5是MycP5的直接相互作用伙伴。









































 京公网安备 11010802027423号
京公网安备 11010802027423号