当前位置:
X-MOL 学术
›
J. Environ. Manag.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamics of Strontium and geochemically correlated elements in soil during washing remediation with eco-complaint chelators.
Journal of Environmental Management ( IF 8.0 ) Pub Date : 2020-01-14 , DOI: 10.1016/j.jenvman.2019.110018 Zinnat A Begum 1 , Ismail M M Rahman 2 , Kento Ishii 3 , Hirofumi Tsukada 2 , Hiroshi Hasegawa 4
Journal of Environmental Management ( IF 8.0 ) Pub Date : 2020-01-14 , DOI: 10.1016/j.jenvman.2019.110018 Zinnat A Begum 1 , Ismail M M Rahman 2 , Kento Ishii 3 , Hirofumi Tsukada 2 , Hiroshi Hasegawa 4
Affiliation
|
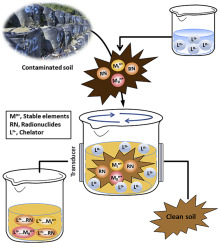
In the study, the dynamics of Sr2+ and geochemically correlated elements (Ca2+, Ba2+, and Y3+) in soil with chelators in the mix (soil to chelator ratio, 1:10; matrix, H2O) were assessed to understand chemical-induced washing remediation of radiogenic waste solids. Specifically, EDTA (2,2',2″,2‴-(ethane-1,2-diyldinitrilo)tetraacetic acid), EDDS (2-[2-(1,2-dicarboxyethylamino)ethylamino]butanedioic acid), GLDA (2-[bis(carboxymethyl)amino]pentanedioic acid), and HIDS (2-(1,2-dicarboxyethylamino)-3-hydroxy-butanedioic acid) are chelators that are used as extractants. The effect of solution pH on chelator-induced extractions of the target elements (t-Es: Sr2+, Ca2+, Ba2+, or Y3+) from soil and stability constants of the t-Es complexes with chelators were used to explain the trends and magnitudes in interactions. Pre- and post-extractive solid-phase speciation was used to define the extent of the competence of each chelator in persuading dissolution of t-Es in the soil. The effects of ultrasonic energy, admixtures of biodegradable chelators, and excess chelators in solution (1:20) were also analyzed on the extractive removal of t-Es from soil. The results indicate that the Sr2+ removal with biodegradable chelators significantly exceeded (approximately 70%) when compared to that of environmentally-persistent EDTA at lower solution pHs and a higher soil to chelator ratio (GLDA > HIDS > EDDS ≈ EDTA). However, the extraction of the geochemically related element was significantly lower.
中文翻译:

生态投诉螯合剂在冲洗修复过程中土壤中锶和地球化学相关元素的动态。
在这项研究中,对土壤中Sr2 +和地球化学相关元素(Ca2 +,Ba2 +和Y3 +)的动力学进行了评估,以混合螯合剂(土壤与螯合剂之比为1:10;基质为H2O)来了解化学诱导的洗涤修复放射性废物固体。具体地,EDTA(2,2',2'',2′-(乙烷-1,2-二基二苯并三)四乙酸),EDDS(2- [2-(1,2-二羧乙基氨基)乙基氨基]丁二酸),GLDA 2- [双(羧甲基)氨基]戊二酸)和HIDS(2-(1,2-二羧乙基氨基)-3-羟基丁二酸)是用作萃取剂的螯合剂。溶液pH值对螯合剂诱导的从土壤中萃取目标元素(t-Es:Sr2 +,Ca2 +,Ba2 +或Y3 +)的影响以及t-Es配合物与螯合剂的稳定常数用于解释趋势和幅度。互动。提取前和提取后的固相形态被用来确定每种螯合剂在说服t-E在土壤中溶解的能力范围。还分析了超声能量,可生物降解螯合剂的混合物和溶液中过量螯合剂(1:20)对土壤中t-Es萃取去除的影响。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。还分析了可降解生物螯合剂的混合物,溶液中过量的螯合剂(1:20)对土壤中t-Es的萃取去除作用。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。还分析了可降解生物螯合剂的混合物,溶液中过量的螯合剂(1:20)对土壤中t-Es的萃取去除作用。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。
更新日期:2020-01-15
中文翻译:

生态投诉螯合剂在冲洗修复过程中土壤中锶和地球化学相关元素的动态。
在这项研究中,对土壤中Sr2 +和地球化学相关元素(Ca2 +,Ba2 +和Y3 +)的动力学进行了评估,以混合螯合剂(土壤与螯合剂之比为1:10;基质为H2O)来了解化学诱导的洗涤修复放射性废物固体。具体地,EDTA(2,2',2'',2′-(乙烷-1,2-二基二苯并三)四乙酸),EDDS(2- [2-(1,2-二羧乙基氨基)乙基氨基]丁二酸),GLDA 2- [双(羧甲基)氨基]戊二酸)和HIDS(2-(1,2-二羧乙基氨基)-3-羟基丁二酸)是用作萃取剂的螯合剂。溶液pH值对螯合剂诱导的从土壤中萃取目标元素(t-Es:Sr2 +,Ca2 +,Ba2 +或Y3 +)的影响以及t-Es配合物与螯合剂的稳定常数用于解释趋势和幅度。互动。提取前和提取后的固相形态被用来确定每种螯合剂在说服t-E在土壤中溶解的能力范围。还分析了超声能量,可生物降解螯合剂的混合物和溶液中过量螯合剂(1:20)对土壤中t-Es萃取去除的影响。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。还分析了可降解生物螯合剂的混合物,溶液中过量的螯合剂(1:20)对土壤中t-Es的萃取去除作用。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。还分析了可降解生物螯合剂的混合物,溶液中过量的螯合剂(1:20)对土壤中t-Es的萃取去除作用。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。结果表明,在较低的溶液pH值和较高的土壤与螯合剂比率(GLDA> HIDS> EDDS≈EDTA)下,与可环境降解的EDTA相比,可生物降解的螯合剂对Sr2 +的去除明显超过(约70%)。但是,地球化学相关元素的提取率明显较低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号