当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multivalent electrolyte induced surface ordering and solution self-assembly in anionic surfactant mixtures: Sodium dodecyl sulfate and sodium diethylene glycol monododecyl sulfate.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.jcis.2020.01.032 Peixun Li 1 , Zi Wang 2 , Kun Ma 1 , Yao Chen 1 , Zifeng Yan 3 , Jeff Penfold 4 , Robert K Thomas 5 , Mario Campana 1 , John R P Webster 1 , Adam Washington 1
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.jcis.2020.01.032 Peixun Li 1 , Zi Wang 2 , Kun Ma 1 , Yao Chen 1 , Zifeng Yan 3 , Jeff Penfold 4 , Robert K Thomas 5 , Mario Campana 1 , John R P Webster 1 , Adam Washington 1
Affiliation
|
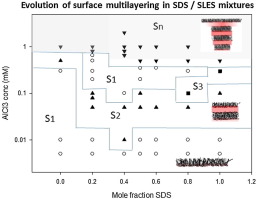
The formation of surface multilayer structures, with the addition of multivalent electrolytes, has been observed in a range of different anionic surfactants; and notably the sodium oxyethylene glycol alkyl sulfate, SAES, and alkyl ester sulfonate, AES, surfactants. The addition of increasing amounts of AlCl3 results in increasing surface layering, with a transition from monolayer to bilayer to ultimately more extended multilayer structures at the interface. The headgroup structures of these SAES and AES surfactants and their hydrophilic / hydrophobic balance give a degree of tolerance to the precipitation induced by multivalent counterions. This was considered to be important factor associated with the multivalent counterion induced surface layering. In this paper the impact of sodium dodecyl sulfate, SDS, an anionic surfactant more susceptible to precipitation in the presence of multivalent counterions, on the surface multilayer formation and solution self-assembly of sodium diethylene glycol monododecyl sulfate, SLES, is explored using surface tension, neutron reflectivity and small angle neutron scattering. The results show that SDS exhibits a similar progressive evolution in surface structures with increasing AlCl3 concentrations, as observed in SLES and related SAES surfactants, and in MES, sodium methyl ester dodecyl sulfonate surfactant. However in the SLES / SDS mixtures the structural evolution is different, and more complex pattern with increasing AlCl3 concentration is observed. The initial transition from monolayer to bilayer / trilayer structures exists, but the surface at higher AlCl3 concentration reverts to monolayer adsorption before extended multilayer structures are formed. Complementary small angle neutron scattering measurements indicate a more complex evolution in the micelle structure which broadly correlates with the surface behaviour. The results illustrate how subtle changes in headgroup structure and packing affect relative counterion binding and hence the surface and solution structures. The results reinforce and extend the observations of related structures on different SAES and AES surfactants, and highlight the opportunity for manipulating surface adsorption behaviour with surfactant mixtures.
中文翻译:

阴离子表面活性剂混合物中的多价电解质诱导的表面有序化和溶液自组装:十二烷基硫酸钠和二甘醇单十二烷基硫酸钠。
在多种不同的阴离子表面活性剂中,观察到在添加多价电解质的情况下表面多层结构的形成。尤其是氧化乙二醇烷基硫酸钠钠SAES和烷基酯磺酸盐AES表面活性剂。增加数量的AlCl3的添加会导致表面分层的增加,并在界面处从单层过渡到双层,最终过渡到更多扩展的多层结构。这些SAES和AES表面活性剂的头基结构及其亲水/疏水平衡为多价抗衡离子诱导的沉淀提供一定程度的耐受性。这被认为是与多价抗衡离子诱导的表面分层相关的重要因素。本文对十二烷基硫酸钠,SDS,使用表面张力,中子反射率和小角度中子散射,探索了一种在多价抗衡离子存在下更易沉淀的阴离子表面活性剂,该表面活性剂是在二乙二醇单十二烷基硫酸钠钠SLES的表面多层形成和溶液自组装上形成的。结果表明,如在SLES和相关的SAES表面活性剂中以及在MES甲基丙烯酸十二烷基磺酸钠表面活性剂中所观察到的,SDS在表面结构中随着AlCl3浓度的增加表现出相似的渐进演化。但是,在SLES / SDS混合物中,结构演变是不同的,并且观察到随着AlCl3浓度增加而出现的更复杂的模式。存在从单层到双层/三层结构的初始过渡,但是在形成扩展的多层结构之前,较高AlCl3浓度的表面会恢复为单层吸附。互补的小角中子散射测量结果表明,胶束结构发生了更为复杂的演化,与表面行为广泛相关。结果说明头基结构和堆积的细微变化如何影响相对的抗衡离子结合,进而影响表面和溶液结构。结果加强并扩展了在不同的SAES和AES表面活性剂上相关结构的观察结果,并强调了利用表面活性剂混合物操纵表面吸附行为的机会。互补的小角中子散射测量结果表明,胶束结构发生了更为复杂的演化,与表面行为广泛相关。结果说明头基结构和堆积的细微变化如何影响相对的抗衡离子结合,进而影响表面和溶液结构。结果加强并扩展了在不同的SAES和AES表面活性剂上相关结构的观察结果,并强调了利用表面活性剂混合物操纵表面吸附行为的机会。互补的小角中子散射测量结果表明,胶束结构发生了更为复杂的演化,与表面行为广泛相关。结果说明头基结构和堆积的细微变化如何影响相对的抗衡离子结合,进而影响表面和溶液结构。结果加强并扩展了在不同的SAES和AES表面活性剂上相关结构的观察结果,并强调了利用表面活性剂混合物操纵表面吸附行为的机会。
更新日期:2020-01-15
中文翻译:

阴离子表面活性剂混合物中的多价电解质诱导的表面有序化和溶液自组装:十二烷基硫酸钠和二甘醇单十二烷基硫酸钠。
在多种不同的阴离子表面活性剂中,观察到在添加多价电解质的情况下表面多层结构的形成。尤其是氧化乙二醇烷基硫酸钠钠SAES和烷基酯磺酸盐AES表面活性剂。增加数量的AlCl3的添加会导致表面分层的增加,并在界面处从单层过渡到双层,最终过渡到更多扩展的多层结构。这些SAES和AES表面活性剂的头基结构及其亲水/疏水平衡为多价抗衡离子诱导的沉淀提供一定程度的耐受性。这被认为是与多价抗衡离子诱导的表面分层相关的重要因素。本文对十二烷基硫酸钠,SDS,使用表面张力,中子反射率和小角度中子散射,探索了一种在多价抗衡离子存在下更易沉淀的阴离子表面活性剂,该表面活性剂是在二乙二醇单十二烷基硫酸钠钠SLES的表面多层形成和溶液自组装上形成的。结果表明,如在SLES和相关的SAES表面活性剂中以及在MES甲基丙烯酸十二烷基磺酸钠表面活性剂中所观察到的,SDS在表面结构中随着AlCl3浓度的增加表现出相似的渐进演化。但是,在SLES / SDS混合物中,结构演变是不同的,并且观察到随着AlCl3浓度增加而出现的更复杂的模式。存在从单层到双层/三层结构的初始过渡,但是在形成扩展的多层结构之前,较高AlCl3浓度的表面会恢复为单层吸附。互补的小角中子散射测量结果表明,胶束结构发生了更为复杂的演化,与表面行为广泛相关。结果说明头基结构和堆积的细微变化如何影响相对的抗衡离子结合,进而影响表面和溶液结构。结果加强并扩展了在不同的SAES和AES表面活性剂上相关结构的观察结果,并强调了利用表面活性剂混合物操纵表面吸附行为的机会。互补的小角中子散射测量结果表明,胶束结构发生了更为复杂的演化,与表面行为广泛相关。结果说明头基结构和堆积的细微变化如何影响相对的抗衡离子结合,进而影响表面和溶液结构。结果加强并扩展了在不同的SAES和AES表面活性剂上相关结构的观察结果,并强调了利用表面活性剂混合物操纵表面吸附行为的机会。互补的小角中子散射测量结果表明,胶束结构发生了更为复杂的演化,与表面行为广泛相关。结果说明头基结构和堆积的细微变化如何影响相对的抗衡离子结合,进而影响表面和溶液结构。结果加强并扩展了在不同的SAES和AES表面活性剂上相关结构的观察结果,并强调了利用表面活性剂混合物操纵表面吸附行为的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号