当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic View of Allosteric Regulation in the Hsp70 Chaperones by J-Domain Cochaperone and Post-Translational Modifications: Computational Analysis of Hsp70 Mechanisms by Exploring Conformational Landscapes and Residue Interaction Networks.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jcim.9b01045 Lindy Astl 1 , Gennady M Verkhivker 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.jcim.9b01045 Lindy Astl 1 , Gennady M Verkhivker 1, 2
Affiliation
|
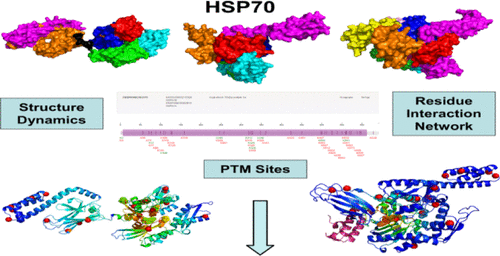
Structural and biochemical studies of Hsp70 chaperones have provided a molecular view of the chaperone biochemical cycle by revealing a complex interplay between allosteric conformational states that controls the feedback loop between stimulation of the adenosinetriphosphatase (ATPase) activity and the substrate release. Allosteric regulation in the Hsp70 chaperones and efficient substrate targeting are mediated by J-domain cochaperones through a dynamic interaction network controlled by the regulatory hotspots. In the current work, we have simulated conformational landscapes and residue interaction networks in the open, closed, and cochaperone-bound DnaK structures. The results of this work have shown that J-domain can selectively enhance direction-specific signal propagation from the substrate-binding domain to the catalytic center and promote the structural environment required for ATP hydrolysis. By employing different network-based approaches, we examined the role and contribution of post-translational modification sites in allosteric regulation of human Hsp70. The central finding of this analysis indicated that conserved phosphorylation sites localized preferentially in the nucleotide-binding domain regions are often aligned with the allosteric control points and serve as effector centers in Hsp70. We have found that cooperation of post-translational modifications sites is based on the governing role of phosphorylation sites in dictating regulatory switching functions, whereas the bulk of acetylation sites can be involved in sensing the long-range signals and executing allosteric changes during the ATPase cycle. The results of this study highlight the important role of phosphorylation sites in exerting control over allosteric changes in Hsp70. The network-centric framework for the analysis of conformational dynamics and chaperone landscapes can explain a range of structural and functional experiments, providing a robust dynamic model of Hsp70 regulation by cochaperones and sites of post-translational modifications.
中文翻译:

通过J-Domain Cochaperone和翻译后修饰对Hsp70伴侣进行变构调节的动态视图:通过探索构象态和残基相互作用网络对Hsp70机理进行的计算分析。
Hsp70分子伴侣的结构和生化研究通过揭示变构构象状态之间复杂的相互作用来提供分子伴侣生化循环的分子观点,该构象状态控制了腺苷三磷酸酶(ATPase)活性刺激与底物释放之间的反馈环。Hsp70分子伴侣中的变构调控和有效的底物靶向是通过调控热点控制的动态相互作用网络由J结构域伴侣分子介导的。在当前的工作中,我们已经模拟了开放,封闭和陪伴酮结合的DnaK结构中的构象态势和残基相互作用网络。这项工作的结果表明,J结构域可以选择性地增强方向特异性信号从底物结合结构域到催化中心的传播,并促进ATP水解所需的结构环境。通过采用不同的基于网络的方法,我们检查了人类Hsp70的变构调节中翻译后修饰位点的作用和贡献。该分析的主要发现表明,优先位于核苷酸结合域区域中的保守磷酸化位点通常与变构控制点对齐,并在Hsp70中充当效应子中心。我们发现,翻译后修饰位点的合作是基于磷酸化位点在决定调控开关功能中的调控作用,而大量的乙酰化位点可参与传感远距离信号并在ATPase循环中执行变构变化。这项研究的结果突出了磷酸化位点在控制Hsp70的变构变化中的重要作用。用于分析构象动力学和分子伴侣态势的以网络为中心的框架可以解释一系列结构和功能实验,提供了由伴侣蛋白和翻译后修饰位点对Hsp70调控的强大动力学模型。
更新日期:2020-01-14
中文翻译:

通过J-Domain Cochaperone和翻译后修饰对Hsp70伴侣进行变构调节的动态视图:通过探索构象态和残基相互作用网络对Hsp70机理进行的计算分析。
Hsp70分子伴侣的结构和生化研究通过揭示变构构象状态之间复杂的相互作用来提供分子伴侣生化循环的分子观点,该构象状态控制了腺苷三磷酸酶(ATPase)活性刺激与底物释放之间的反馈环。Hsp70分子伴侣中的变构调控和有效的底物靶向是通过调控热点控制的动态相互作用网络由J结构域伴侣分子介导的。在当前的工作中,我们已经模拟了开放,封闭和陪伴酮结合的DnaK结构中的构象态势和残基相互作用网络。这项工作的结果表明,J结构域可以选择性地增强方向特异性信号从底物结合结构域到催化中心的传播,并促进ATP水解所需的结构环境。通过采用不同的基于网络的方法,我们检查了人类Hsp70的变构调节中翻译后修饰位点的作用和贡献。该分析的主要发现表明,优先位于核苷酸结合域区域中的保守磷酸化位点通常与变构控制点对齐,并在Hsp70中充当效应子中心。我们发现,翻译后修饰位点的合作是基于磷酸化位点在决定调控开关功能中的调控作用,而大量的乙酰化位点可参与传感远距离信号并在ATPase循环中执行变构变化。这项研究的结果突出了磷酸化位点在控制Hsp70的变构变化中的重要作用。用于分析构象动力学和分子伴侣态势的以网络为中心的框架可以解释一系列结构和功能实验,提供了由伴侣蛋白和翻译后修饰位点对Hsp70调控的强大动力学模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号