当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Surface Dynamics for Creating Highly Active Ru Sites for Ammonia Synthesis: Accumulation of a Low-Crystalline, Oxygen-Deficient Nanofraction
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/acssuschemeng.9b06299 Katsutoshi Sato 1, 2 , Shin-ichiro Miyahara 2 , Yuta Ogura 2 , Kotoko Tsujimaru 3 , Yuichiro Wada 3 , Takaaki Toriyama 4 , Tomokazu Yamamoto 5 , Syo Matsumura 4, 5 , Katsutoshi Nagaoka 2
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-01-22 , DOI: 10.1021/acssuschemeng.9b06299 Katsutoshi Sato 1, 2 , Shin-ichiro Miyahara 2 , Yuta Ogura 2 , Kotoko Tsujimaru 3 , Yuichiro Wada 3 , Takaaki Toriyama 4 , Tomokazu Yamamoto 5 , Syo Matsumura 4, 5 , Katsutoshi Nagaoka 2
Affiliation
|
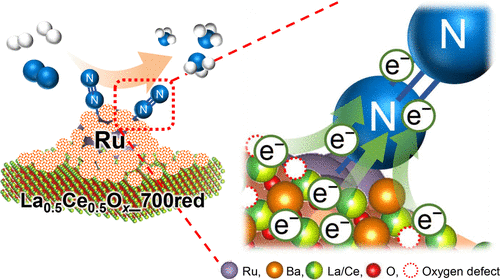
To mitigate global problems related to energy and global warming, it is helpful to develop an ammonia synthesis process using catalysts that are highly active under mild conditions. Here we show that the ammonia synthesis activity per weight of catalyst of Ru/Ba/LaCeOx, prereduced at 700 °C, is the highest among reported oxide-supported Ru catalysts, 52.3 mmol h–1 gcat–1 at 350 °C, 1.0 MPa. The turnover frequency of Ru/Ba/LaCeOx at 350 °C was more than 8 times that of Cs+/Ru/MgO, which is a well-known active catalyst used as a benchmark; furthermore, hydrogen poisoning, a typical drawback for oxide-supported Ru catalysts, was effectively suppressed. Scanning transmission electron microscopy observations with energy dispersive X-ray spectrometry and electron energy loss spectroscopy analysis revealed that a low-crystalline, oxygen-deficient nanofraction including Ba2+, Ce3+, and La3+ had accumulated on the Ru particles. This unique structure was obtained by exploiting the surface dynamics of alkaline earth compounds and thermostable rare earth oxides that contain redox-active atoms during the reduction at an unusually high temperature. The nanofraction showed strong electron-donating ability because of the strong basicity of the included cations, removal of carbonate, and formation of oxygen defect sites that eliminated electron-withdrawing O2– anions from the interface between the nanofraction and Ru atom. Electrons were therefore effectively donated to antibonding π-orbitals of the N2 molecules via Ru in contact with the nanofraction, and N≡N triple bond cleavage, which is the rate-determining step for ammonia synthesis, was promoted.
中文翻译:

用于创建氨合成的高活性Ru位的表面动力学:低结晶度,缺氧的纳米级分的积累
为了减轻与能源和全球变暖有关的全球性问题,开发使用在温和条件下具有高活性的催化剂的氨合成工艺很有帮助。在这里我们表明,Ru / Ba / LaCeO x催化剂每单位重量的氨合成活性在700°C时已预先降低,在报道的氧化物负载的Ru催化剂中最高,在350°C时为52.3 mmol h –1 g cat –1 1.0兆帕。Ru / Ba / LaCeO x在350°C下的周转频率是Cs +的周转频率的8倍以上/ Ru / MgO,它是众所周知的用作基准的活性催化剂;此外,有效地抑制了氢中毒,氢中毒是氧化物负载的Ru催化剂的典型缺点。扫描透射电子显微镜观察与能量色散X射线光谱法和电子能量损失谱分析表明,低结晶度,缺氧的纳米级组分包括Ba 2 +,Ce 3+和La 3+积累在Ru颗粒上 这种独特的结构是通过在异常高温下还原过程中利用碱土化合物和包含氧化还原活性原子的热稳定稀土氧化物的表面动力学而获得的。由于所含阳离子的强碱性,碳酸盐的去除以及氧缺陷位点的形成消除了从纳米级分与Ru原子之间的界面吸收电子的O 2-阴离子,因此纳米级分显示出强大的给电子能力。因此,通过与纳米级接触的Ru有效地将电子赠予N 2分子的反键π轨道,并促进了N≡N三键裂解,这是氨合成的决定速率的步骤。
更新日期:2020-01-23
中文翻译:

用于创建氨合成的高活性Ru位的表面动力学:低结晶度,缺氧的纳米级分的积累
为了减轻与能源和全球变暖有关的全球性问题,开发使用在温和条件下具有高活性的催化剂的氨合成工艺很有帮助。在这里我们表明,Ru / Ba / LaCeO x催化剂每单位重量的氨合成活性在700°C时已预先降低,在报道的氧化物负载的Ru催化剂中最高,在350°C时为52.3 mmol h –1 g cat –1 1.0兆帕。Ru / Ba / LaCeO x在350°C下的周转频率是Cs +的周转频率的8倍以上/ Ru / MgO,它是众所周知的用作基准的活性催化剂;此外,有效地抑制了氢中毒,氢中毒是氧化物负载的Ru催化剂的典型缺点。扫描透射电子显微镜观察与能量色散X射线光谱法和电子能量损失谱分析表明,低结晶度,缺氧的纳米级组分包括Ba 2 +,Ce 3+和La 3+积累在Ru颗粒上 这种独特的结构是通过在异常高温下还原过程中利用碱土化合物和包含氧化还原活性原子的热稳定稀土氧化物的表面动力学而获得的。由于所含阳离子的强碱性,碳酸盐的去除以及氧缺陷位点的形成消除了从纳米级分与Ru原子之间的界面吸收电子的O 2-阴离子,因此纳米级分显示出强大的给电子能力。因此,通过与纳米级接触的Ru有效地将电子赠予N 2分子的反键π轨道,并促进了N≡N三键裂解,这是氨合成的决定速率的步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号