当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Chemical Modification of Cysteine Residues on Protein Surfaces Focusing on Local Environment around the Conjugation Site.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.bioconjchem.9b00869 Teruyuki Miyake 1 , Ryosei Tamaki 1 , Moeko Asanuma 1 , Yoji Fukada 1 , Shun Hirota 1 , Takashi Matsuo 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-01-27 , DOI: 10.1021/acs.bioconjchem.9b00869 Teruyuki Miyake 1 , Ryosei Tamaki 1 , Moeko Asanuma 1 , Yoji Fukada 1 , Shun Hirota 1 , Takashi Matsuo 1
Affiliation
|
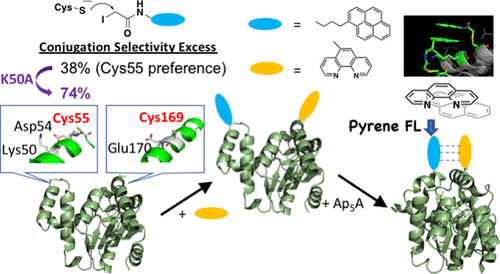
For chemical modification of cysteines in a protein, the regioselectivity among cysteine residues on the protein surface is an issue to be considered. To elucidate the determinants of cysteine reactivities on protein surfaces, we have investigated the chemical modification of the adenylate kinase A55C/C77S/V169C mutant as an experimental model. Although Cys55 and Cys169 are commonly located on the protein surface, Cys55 showed the ca. 3-6-fold higher reactivity compared to Cys169 in a reaction with a pyrene derivative. By a further conjugation of a phenanthroline derivative into the vacant Cys thiol, fluorescence quenching was attained by a pyrene-phenanthroline interaction that occurred by the conformational change of the protein. The K50A mutation further enhanced the regioselectivity of pyrene conjugation in Cys55, which is attributed to the effects of structural flexibility in the vicinity of Cys55 on its reactivity. To regioselectively conjugate different types of synthetic molecules onto the surface of a protein, perturbation in the local structural flexibility around the conjugation sites will be a useful strategy.
中文翻译:

半胱氨酸残基在蛋白质表面的区域选择性化学修饰,重点在于缀合位点周围的局部环境。
对于蛋白质中的半胱氨酸的化学修饰,蛋白质表面上半胱氨酸残基之间的区域选择性是要考虑的问题。为了阐明在蛋白质表面上半胱氨酸反应性的决定因素,我们研究了腺苷酸激酶A55C / C77S / V169C突变体的化学修饰作为实验模型。尽管Cys55和Cys169通常位于蛋白质表面,但Cys55显示出ca。在与a衍生物的反应中,与Cys169相比,反应活性高3-6倍。通过将菲咯啉衍生物进一步缀合到空的Cys硫醇中,通过蛋白质构象变化发生的that-菲咯啉相互作用实现了荧光猝灭。K50A突变进一步增强了Cys55中of结合的区域选择性,这归因于Cys55附近的结构柔性对其反应性的影响。为了将不同类型的合成分子区域选择性地缀合到蛋白质的表面,在缀合位点周围的局部结构柔韧性的扰动将是有用的策略。
更新日期:2020-01-27
中文翻译:

半胱氨酸残基在蛋白质表面的区域选择性化学修饰,重点在于缀合位点周围的局部环境。
对于蛋白质中的半胱氨酸的化学修饰,蛋白质表面上半胱氨酸残基之间的区域选择性是要考虑的问题。为了阐明在蛋白质表面上半胱氨酸反应性的决定因素,我们研究了腺苷酸激酶A55C / C77S / V169C突变体的化学修饰作为实验模型。尽管Cys55和Cys169通常位于蛋白质表面,但Cys55显示出ca。在与a衍生物的反应中,与Cys169相比,反应活性高3-6倍。通过将菲咯啉衍生物进一步缀合到空的Cys硫醇中,通过蛋白质构象变化发生的that-菲咯啉相互作用实现了荧光猝灭。K50A突变进一步增强了Cys55中of结合的区域选择性,这归因于Cys55附近的结构柔性对其反应性的影响。为了将不同类型的合成分子区域选择性地缀合到蛋白质的表面,在缀合位点周围的局部结构柔韧性的扰动将是有用的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号