当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Interaction of Redox Active Organic Molecules and Solvents with the Pristine and Defective Graphene Surfaces from Density Functional Theory
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jpcc.9b10403 Jason D. Howard 1, 2 , Rajeev S. Assary 1, 2 , Larry A. Curtiss 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-24 , DOI: 10.1021/acs.jpcc.9b10403 Jason D. Howard 1, 2 , Rajeev S. Assary 1, 2 , Larry A. Curtiss 1, 2
Affiliation
|
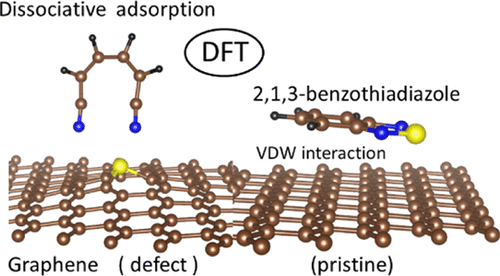
A systematic study is reported on the interaction of two representative redox active organic molecules and two solvent molecules with pristine and defective graphene surfaces as a model of an electrode surface of a redox flow battery (RFB). The redox active molecules include a catholyte, 2,5-di-tert-butyl-1,4-dimethoxybenzene (DDB), and an anolyte molecule, benzothiadiazole (BTZ), and the solvent molecules include acetonitrile (MeCN) and ethylene carbonate (EC). The graphene defects investigated include a single vacancy, double vacancy, zigzag step edge, and armchair step edge. Computations suggest that the interactions of all molecules with a pristine graphene surface are relatively weak (0.2 to 0.8 eV) and dominated by van der Waals effects; therefore, these molecules are chemically stable upon interacting with pristine nondefective graphene. The BTZ, DDB, MeCN, and EC molecules interact strongly (1.5 to 5.5 eV) with the single vacancy and zigzag step edges of graphene that leads to the possible decomposition of the molecules with strength of interaction in the order of MeCN > BTZ > DDB > EC and MeCN > EC > BTZ > DDB, respectively. Calculations show that the BTZ, DDB, MeCN, and EC molecules interact less strongly (0.2 to 1.4 eV) to the double vacancy and armchair step edge than to the single vacancy and zigzag step edge. The binding energies of the molecules were significantly reduced when interacting with the passivated defects, suggesting that the passivation of the defects could help prevent unwanted chemical interactions between the neutral molecules and the electrode surface. In all organic RFBs, one of the crucial bottlenecks is the stability of the constituent molecules with the electrode material, and this study provides insights into the chemical interaction of selected candidate species with a model carbon electrode.
中文翻译:

从密度泛函理论洞察氧化还原活性有机分子和溶剂与原始和有缺陷的石墨烯表面的相互作用
有系统的研究报道了两个代表性的氧化还原活性有机分子和两个溶剂分子与原始和有缺陷的石墨烯表面的相互作用,作为氧化还原液流电池(RFB)电极表面的模型。氧化还原活性分子包括一个阴极,2,5-二-叔-1,4-丁基二甲氧基苯(DDB)和阳极液分子苯并噻二唑(BTZ),溶剂分子包括乙腈(MeCN)和碳酸亚乙酯(EC)。研究的石墨烯缺陷包括单空位,双空位,之字形台阶边缘和扶手椅台阶边缘。计算表明,所有分子与原始石墨烯表面的相互作用都相对较弱(0.2至0.8 eV),并且受范德华效应支配。因此,这些分子在与原始的无缺陷石墨烯相互作用时具有化学稳定性。BTZ,DDB,MeCN和EC分子与石墨烯的单个空位和之字形台阶边缘发生强烈相互作用(1.5至5.5 eV),从而导致分子可能以MeCN> BTZ> DDB的相互作用强度分解> EC和MeCN> EC> BTZ> DDB,分别。计算表明,BTZ,DDB,MeCN和EC分子与双空位和扶手椅台阶边缘的相互作用比单空和之字形台阶边缘的相互作用弱(0.2到1.4 eV)。与钝化缺陷相互作用时,分子的结合能显着降低,这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。4 eV)到双空位和扶手椅台阶边缘比到单空位和锯齿台阶边缘。与钝化缺陷相互作用时,分子的结合能显着降低,这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。4 eV)到双空位和扶手椅台阶边缘比到单空位和锯齿台阶边缘。与钝化缺陷相互作用时,分子的结合能显着降低,这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。
更新日期:2020-01-24
中文翻译:

从密度泛函理论洞察氧化还原活性有机分子和溶剂与原始和有缺陷的石墨烯表面的相互作用
有系统的研究报道了两个代表性的氧化还原活性有机分子和两个溶剂分子与原始和有缺陷的石墨烯表面的相互作用,作为氧化还原液流电池(RFB)电极表面的模型。氧化还原活性分子包括一个阴极,2,5-二-叔-1,4-丁基二甲氧基苯(DDB)和阳极液分子苯并噻二唑(BTZ),溶剂分子包括乙腈(MeCN)和碳酸亚乙酯(EC)。研究的石墨烯缺陷包括单空位,双空位,之字形台阶边缘和扶手椅台阶边缘。计算表明,所有分子与原始石墨烯表面的相互作用都相对较弱(0.2至0.8 eV),并且受范德华效应支配。因此,这些分子在与原始的无缺陷石墨烯相互作用时具有化学稳定性。BTZ,DDB,MeCN和EC分子与石墨烯的单个空位和之字形台阶边缘发生强烈相互作用(1.5至5.5 eV),从而导致分子可能以MeCN> BTZ> DDB的相互作用强度分解> EC和MeCN> EC> BTZ> DDB,分别。计算表明,BTZ,DDB,MeCN和EC分子与双空位和扶手椅台阶边缘的相互作用比单空和之字形台阶边缘的相互作用弱(0.2到1.4 eV)。与钝化缺陷相互作用时,分子的结合能显着降低,这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。4 eV)到双空位和扶手椅台阶边缘比到单空位和锯齿台阶边缘。与钝化缺陷相互作用时,分子的结合能显着降低,这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。4 eV)到双空位和扶手椅台阶边缘比到单空位和锯齿台阶边缘。与钝化缺陷相互作用时,分子的结合能显着降低,这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。这表明缺陷的钝化可以帮助防止中性分子与电极表面之间发生有害的化学相互作用。在所有有机RFB中,关键的瓶颈之一是组成分子与电极材料的稳定性,这项研究提供了对选定候选物种与模型碳电极之间化学相互作用的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号