Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
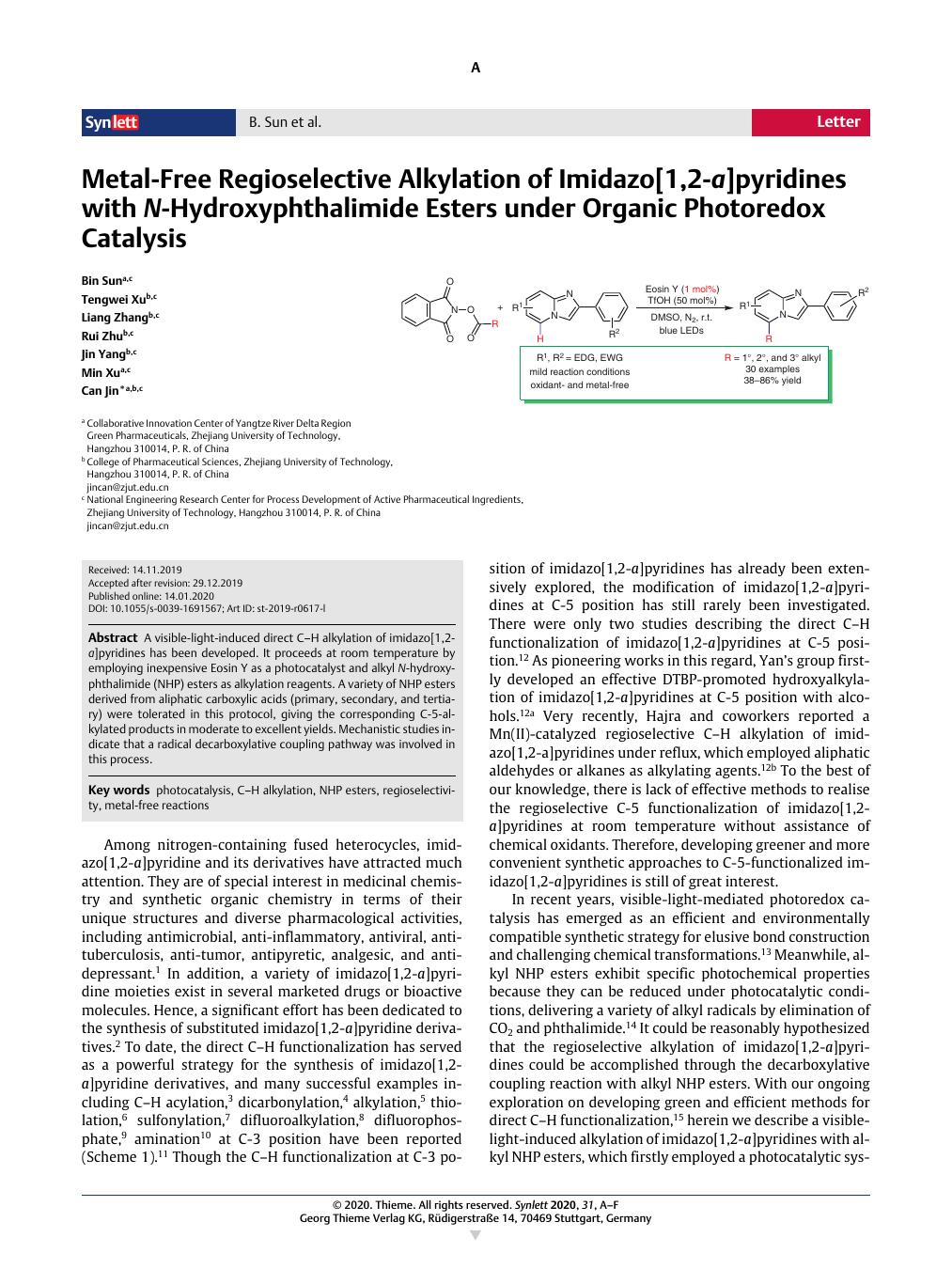
Metal-Free Regioselective Alkylation of Imidazo[1,2-a]pyridines with N-Hydroxyphthalimide Esters under Organic Photoredox Catalysis
Synlett ( IF 1.7 ) Pub Date : 2020-01-14 , DOI: 10.1055/s-0039-1691567 Bin Sun 1, 2 , Tengwei Xu 2, 3 , Liang Zhang 2, 3 , Rui Zhu 2, 3 , Jin Yang 2, 3 , Min Xu 1, 2 , Can Jin 1, 2, 3
Synlett ( IF 1.7 ) Pub Date : 2020-01-14 , DOI: 10.1055/s-0039-1691567 Bin Sun 1, 2 , Tengwei Xu 2, 3 , Liang Zhang 2, 3 , Rui Zhu 2, 3 , Jin Yang 2, 3 , Min Xu 1, 2 , Can Jin 1, 2, 3
Affiliation
|
A visible-light-induced direct C–H alkylation of imidazo[1,2-a]pyridines has been developed. It proceeds at room temperature by employing inexpensive Eosin Y as a photocatalyst and alkyl N-hydroxyphthalimide (NHP) esters as alkylation reagents. A variety of NHP esters derived from aliphatic carboxylic acids (primary, secondary, and tertiary) were tolerated in this protocol, giving the corresponding C-5-alkylated products in moderate to excellent yields. Mechanistic studies indicate that a radical decarboxylative coupling pathway was involved in this process.
中文翻译:

有机光氧化还原催化下咪唑并[1,2-a]吡啶与N-羟基邻苯二甲酰亚胺酯的无金属区域选择性烷基化
已开发出可见光诱导的咪唑并 [1,2-a] 吡啶直接 C-H 烷基化。它通过使用廉价的曙红 Y 作为光催化剂和烷基 N-羟基邻苯二甲酰亚胺 (NHP) 酯作为烷基化试剂在室温下进行。该协议允许使用源自脂肪族羧酸(初级、二级和三级)的各种 NHP 酯,从而以中等至极好的收率提供相应的 C-5 烷基化产品。机理研究表明,该过程涉及自由基脱羧偶联途径。
更新日期:2020-01-14
中文翻译:

有机光氧化还原催化下咪唑并[1,2-a]吡啶与N-羟基邻苯二甲酰亚胺酯的无金属区域选择性烷基化
已开发出可见光诱导的咪唑并 [1,2-a] 吡啶直接 C-H 烷基化。它通过使用廉价的曙红 Y 作为光催化剂和烷基 N-羟基邻苯二甲酰亚胺 (NHP) 酯作为烷基化试剂在室温下进行。该协议允许使用源自脂肪族羧酸(初级、二级和三级)的各种 NHP 酯,从而以中等至极好的收率提供相应的 C-5 烷基化产品。机理研究表明,该过程涉及自由基脱羧偶联途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号