当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adhesion and Surface Layers on Silicon Anodes Suppress Formation of c-Li3.75Si and Solid-Electrolyte Interphase
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-01-14 00:00:00 , DOI: 10.1021/acsaem.9b02090 Hezhen Xie 1, 2 , Sayed Youssef Sayed 1, 2 , W. Peter Kalisvaart 1, 2 , Simon J. Schaper 3 , Peter Müller-Buschbaum 3, 4 , Erik J. Luber 1, 2 , Brian C. Olsen 1, 2 , Martin Haese 5 , Jillian M. Buriak 1, 2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-01-14 00:00:00 , DOI: 10.1021/acsaem.9b02090 Hezhen Xie 1, 2 , Sayed Youssef Sayed 1, 2 , W. Peter Kalisvaart 1, 2 , Simon J. Schaper 3 , Peter Müller-Buschbaum 3, 4 , Erik J. Luber 1, 2 , Brian C. Olsen 1, 2 , Martin Haese 5 , Jillian M. Buriak 1, 2
Affiliation
|
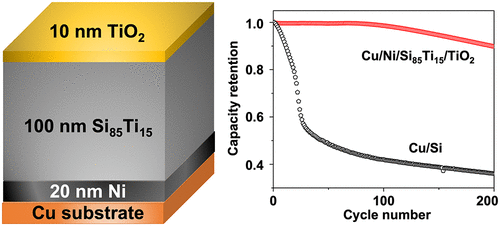
The formation of c-Li3.75Si is known to be detrimental to silicon anodes in lithium-ion batteries. To suppress the formation of this crystalline phase and improve the electrochemical performance of Si-based anodes, three approaches were amalgamated: addition of a nickel adhesion sublayer, alloying of the silicon with titanium, and addition of either carbon or TiO2 as a capping layer. The silicon-based films were analyzed by a suite of methods, including scanning electron microscopy (SEM) and a variety of electrochemical techniques, as well as X-ray photoelectron spectroscopy (XPS) to provide insights into the composition of the resulting solid-electrolyte interphase (SEI). A nickel adhesion layer decreased the extent of delamination of the silicon from the underlying copper substrate, compared to Si deposited directly on Cu, which resulted in less capacity loss. Alloying of silicon with titanium (85% silicon, 15% titanium) further increased the stability. Finally, capping these multilayer electrodes with either a thin 10 nm layer of carbon or TiO2 resulted in the best electrode behavior and lowest cumulative relative irreversible capacity. TiO2 is slightly more effective in enhancing the capacity retention, most likely due to differences in the resulting solid-electrolyte interphase (SEI). The combination of an adhesion layer, alloying, and surface coatings shows a cumulative suppression of the formation of c-Li3.75Si and SEI, resulting in the greatest improvement of capacity retention when all three are incorporated together. However, these strategies appear to only delay the onset of the c-Li3.75Si phase; eventually, the c-Li3.75Si phase will form, and at that point, the capacity degradation rate of all the electrodes becomes similar.
中文翻译:

硅阳极上的粘附和表面层抑制c -Li 3.75 Si和固体电解质界面的形成
已知c -Li 3.75 Si的形成不利于锂离子电池中的硅阳极。为了抑制这种晶相的形成并改善硅基阳极的电化学性能,采用了三种方法:添加镍粘附子层,将硅与钛合金化以及添加碳或TiO 2作为覆盖层。通过一系列方法对硅基薄膜进行了分析,包括扫描电子显微镜(SEM)和各种电化学技术以及X射线光电子能谱(XPS),以深入了解所得固体电解质的组成相间(SEI)。与直接沉积在Cu上的Si相比,镍粘附层减少了硅从下面的铜基板上的分层程度,从而减少了容量损失。硅与钛的合金化(85%的硅,15%的钛)进一步提高了稳定性。最后,用碳或TiO 2的10 nm薄层覆盖这些多层电极会导致最佳的电极性能和最低的相对不可逆累积容量。二氧化钛2在提高容量保持力方面稍微更有效,这很可能是由于所得的固体电解质界面(SEI)不同。粘合层,合金化层和表面涂层的组合显示出对c -Li 3.75 Si和SEI形成的累积抑制,当将所有三种结合在一起时,可最大程度地改善容量保持率。然而,这些策略似乎仅延迟了c -Li 3.75 Si相的出现。最终,将形成c -Li 3.75 Si相,到那时,所有电极的容量衰减率将变得相似。
更新日期:2020-01-14
中文翻译:

硅阳极上的粘附和表面层抑制c -Li 3.75 Si和固体电解质界面的形成
已知c -Li 3.75 Si的形成不利于锂离子电池中的硅阳极。为了抑制这种晶相的形成并改善硅基阳极的电化学性能,采用了三种方法:添加镍粘附子层,将硅与钛合金化以及添加碳或TiO 2作为覆盖层。通过一系列方法对硅基薄膜进行了分析,包括扫描电子显微镜(SEM)和各种电化学技术以及X射线光电子能谱(XPS),以深入了解所得固体电解质的组成相间(SEI)。与直接沉积在Cu上的Si相比,镍粘附层减少了硅从下面的铜基板上的分层程度,从而减少了容量损失。硅与钛的合金化(85%的硅,15%的钛)进一步提高了稳定性。最后,用碳或TiO 2的10 nm薄层覆盖这些多层电极会导致最佳的电极性能和最低的相对不可逆累积容量。二氧化钛2在提高容量保持力方面稍微更有效,这很可能是由于所得的固体电解质界面(SEI)不同。粘合层,合金化层和表面涂层的组合显示出对c -Li 3.75 Si和SEI形成的累积抑制,当将所有三种结合在一起时,可最大程度地改善容量保持率。然而,这些策略似乎仅延迟了c -Li 3.75 Si相的出现。最终,将形成c -Li 3.75 Si相,到那时,所有电极的容量衰减率将变得相似。







































 京公网安备 11010802027423号
京公网安备 11010802027423号