当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Electrocatalytic Interface for Ambient Ammonia Synthesis
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acsenergylett.9b02679 Lin Hu 1 , Zhuo Xing 2 , Xiaofeng Feng 1, 2, 3
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-01-14 , DOI: 10.1021/acsenergylett.9b02679 Lin Hu 1 , Zhuo Xing 2 , Xiaofeng Feng 1, 2, 3
Affiliation
|
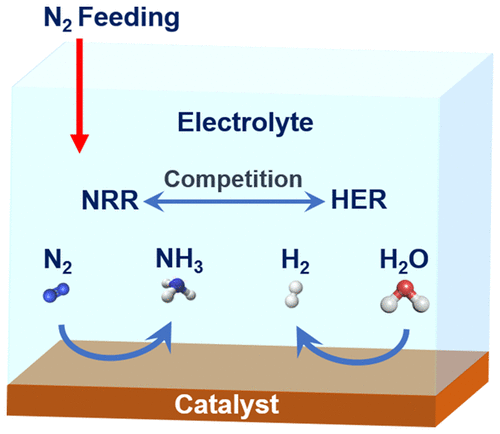
Electrochemical reduction of N2 to NH3 under ambient conditions can enable an alternative approach for sustainable, distributed production of NH3 when powered by renewable electricity. However, the development of such a process has been hindered by the lack of efficient electrocatalysts for the N2 reduction reaction (NRR) because of the barrier for N2 activation and the competing hydrogen evolution reaction (HER). Here, we highlight some mechanistic insights into the electrode–electrolyte interface for NH3 electrosynthesis, where the NRR and HER compete for the available protons, electrons, and catalytic surface sites. The competition even dominates the electrokinetics of NRR, so that the NRR activity typically declines at relatively higher overpotentials. Methods are thus proposed to mitigate the competition from the HER and boost the NRR activity and selectivity, including optimizing the electrolyte, revealing structure–activity relationships for rational catalyst design, and developing gas-diffusion-electrode flow cells with a controlled local liquid/gas environment for NRR electrolysis.
中文翻译:

了解环境氨合成的电催化界面
在环境条件下将N 2电化学还原为NH 3可以为使用可再生电力驱动的NH 3可持续,分布式生产提供一种替代方法。然而,由于N 2活化和竞争性放氢反应(HER)的障碍,缺乏用于N 2还原反应(NRR)的有效电催化剂阻碍了这种方法的发展。在这里,我们重点介绍了NH 3的电极-电解质界面的一些机械原理。电合成,其中NRR和HER竞争可用的质子,电子和催化表面位点。竞争甚至主导了NRR的电动学,因此NRR活性通常在相对较高的超电势下下降。因此,提出了减轻HER竞争并提高NRR活性和选择性的方法,包括优化电解质,揭示合理的催化剂设计的结构-活性关系以及开发具有可控局部液体/气体的气体扩散电极流通池。 NRR电解的环境。
更新日期:2020-01-15
中文翻译:

了解环境氨合成的电催化界面
在环境条件下将N 2电化学还原为NH 3可以为使用可再生电力驱动的NH 3可持续,分布式生产提供一种替代方法。然而,由于N 2活化和竞争性放氢反应(HER)的障碍,缺乏用于N 2还原反应(NRR)的有效电催化剂阻碍了这种方法的发展。在这里,我们重点介绍了NH 3的电极-电解质界面的一些机械原理。电合成,其中NRR和HER竞争可用的质子,电子和催化表面位点。竞争甚至主导了NRR的电动学,因此NRR活性通常在相对较高的超电势下下降。因此,提出了减轻HER竞争并提高NRR活性和选择性的方法,包括优化电解质,揭示合理的催化剂设计的结构-活性关系以及开发具有可控局部液体/气体的气体扩散电极流通池。 NRR电解的环境。











































 京公网安备 11010802027423号
京公网安备 11010802027423号